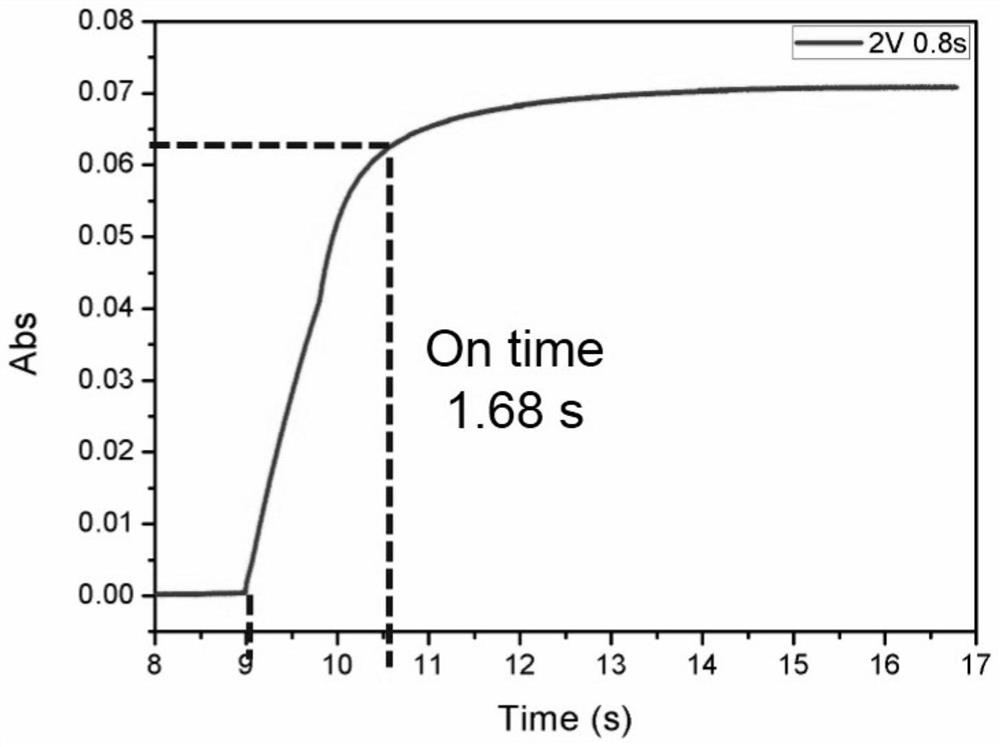
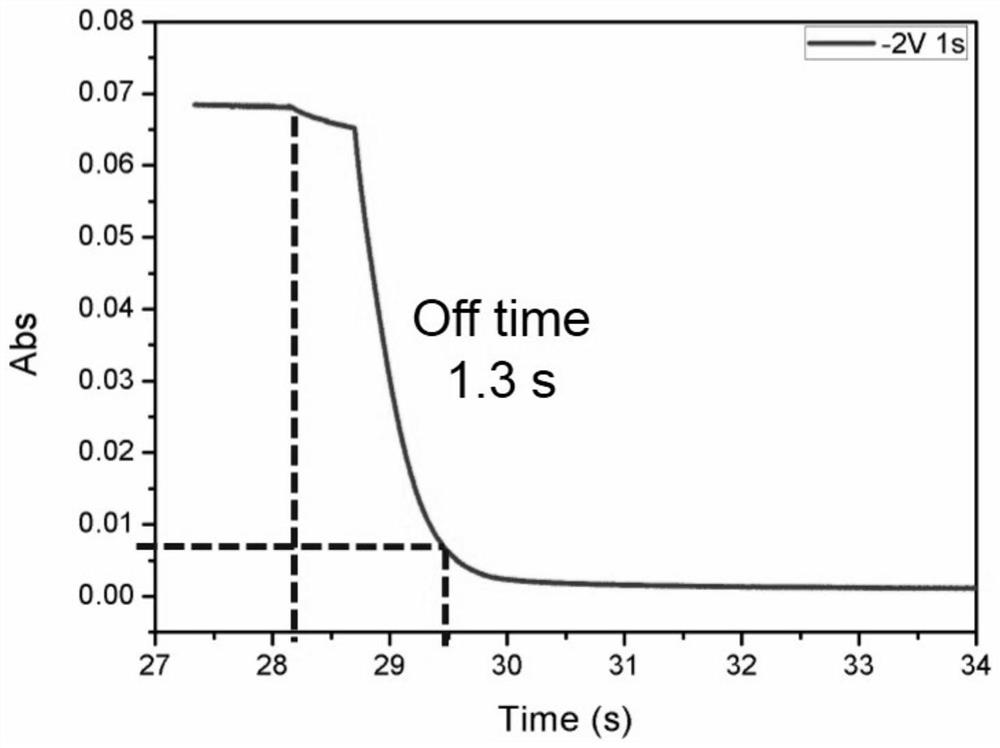
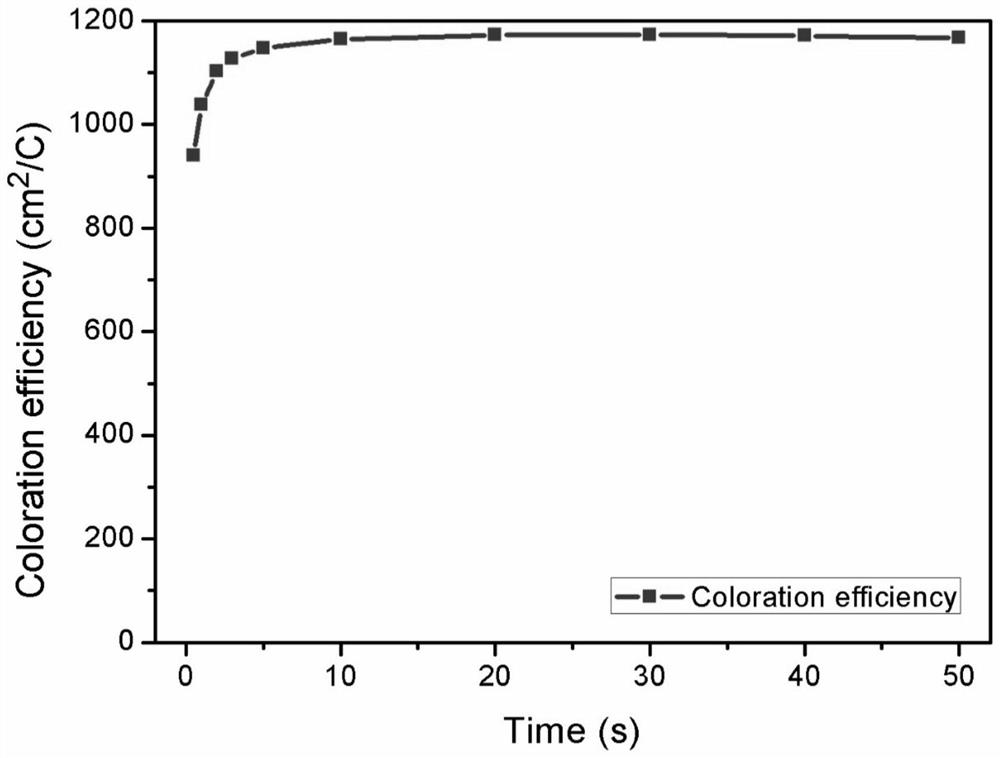
Electrochromic materials based on electroacid action and preparation of electrochromic devices
A technology of electrochromic materials and electrochromic devices, applied in the field of materials, can solve the problems of low efficiency and speed of electrochromic, color is not easy to fade, fading time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] In this embodiment, formula II-1 is used as the electrochromic molecule, 1-butyl-3-methylimidazolium hexafluorophosphate is used as the electrolyte, the two are mixed as the electrochromic material, acetonitrile is used as the solvent, and poly Methyl methacrylate was used as the solid medium. Wherein formula Ⅱ-1 is R in formula Ⅱ 1 , R 3 is N,N-diethyl, R 2 , R 4 , R 5 , R 6 is hydrogen, Y is oxygen, Ar is a benzene ring, Z 1 ,Z 2 It is methyl, and its structural formula is shown in formula II-1. Preparation of electrochromic medium solution: Weigh 1.7g polymethyl methacrylate, 0.1mL (0.5mmol / L) 1-butyl-3-methyl Imidazolium hexafluorophosphate as the electrolyte, 3g (5mmol / L) I-1, and dilute to 10mL with acetonitrile.
[0040]
[0041] Preparation of electrochromic devices: Spin-coat the above-mentioned electrochromic medium solution onto a clean ITO-1 electrode, and then assemble it into an electrochromic device. Under the condition of positive voltage +1.2...
Embodiment 2
[0043] In this embodiment, I-1 is used as the electrochromic molecule, tetrabutylammonium hexafluorophosphate is used as the electrolyte, the two are mixed as the electrochromic material, chloroform is used as the solvent, and polystyrene is used as the solid medium. Wherein formula I-1 is R in general formula I 1 is methyl, R 2 is aminophenyl, R 3 is N,N-diethyl, R 4 , R 5 , R 6 is hydrogen, X is amide, Y is oxygen, Ar is a benzene ring, Z 1 ,Z 2 It is a methyl group, and its structural formula is shown in formula I-1. Preparation of electrochromic medium solution: Weigh 1.7g polystyrene, 0.7g (1.8mmol / L) tetrabutyl ammonium hexafluorophosphate, 0.3g (0.4 mmol / L) I-1, dilute to 10 mL with chloroform.
[0044]
[0045] Preparation of electrochromic device: Spin-coat the above electrochromic medium solution onto a clean ITO-1 electrode, and then assemble it into an electrochromic device. The color becomes transparent to black; under the negative voltage of -1.0V, the...
Embodiment 3
[0047] In this embodiment, formula VI-1 is used as the electrochromic molecule, lithium perchlorate is used as the electrolyte, the two are mixed as the electrochromic material, acetone is used as the solvent, and polyethylene is used as the solid medium. Wherein formula Ⅵ-1 is R in formula Ⅵ 1 , R 3 is aminoethyl, R 2 , R 4 , R 5 , R 6 is hydrogen, Y is dimethyl silicon, Ar is a naphthalene ring, Z 1 It is methyl, and its structural formula is shown in formula VI-1. Electrochromic medium solution preparation: Weigh 1.7g polyethylene, 0.8g (7.5mmol / L) lithium perchlorate, 0.4g (0.6mmol / L) For Ⅵ-1 molecule, dilute to 10 mL with acetone.
[0048]
[0049] Preparation of electrochromic device: Spin-coat the above electrochromic medium solution onto a clean ITO-1 electrode, and then assemble it into an electrochromic device. The electrochromic device is under a positive voltage of +1.2V, and the device consists of Colorless and transparent to blue; under negative voltage...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com