Dehalogenated peroxidase and preparation method and application thereof
A dehalogenated peroxide and reaction technology, applied in the field of ultra-high-efficiency artificial dehalogenated peroxidase and its preparation, to achieve the effect of improving affinity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
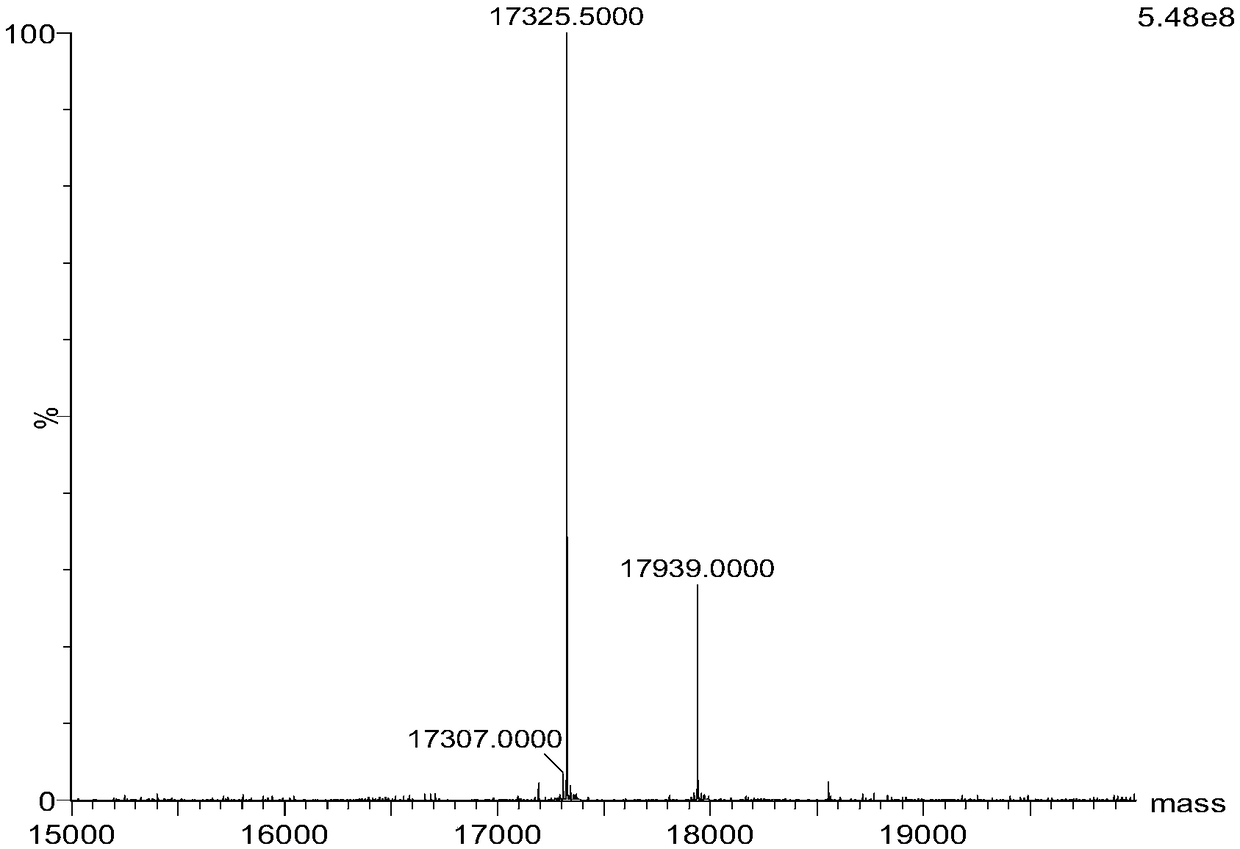
[0035] Based on genetic engineering and protein engineering, using site-directed mutagenesis technology, tyrosine was introduced at position 43 near the heme active center of myoglobin, and histidine at position 64 was mutated into aspartic acid at the same time. Expressed in BL21(DE3), and separated and purified by guanidine hydrochloride denaturation dialysis, ion exchange, column chromatography and other methods to obtain F43Y / H64D Mb mutant protein. The protein molecular weight was shown by mass spectrometry ( figure 1 ).
[0036] The amino acid sequence (SEQ ID NO.1) of F43Y / H64D Mb is as follows:
[0037] MVLSEGEWQLVLHVWAKVEADVAGHGQDILIRLFKSHPETLEKYDRFKHLKTEAEMKASEDLKKDGVTVLTALGAILKKKGHHEAELKPLAQSHATKHKIPIKYLEFISEAIIHVLHSRHPGDFGADAQGAMNKALELFRKDIAAKYKELGYQG
Embodiment 2
[0039] 4 μM F43Y / H64D Mb was prepared in 100 mM phosphate buffer (pH 7.0). Meanwhile, prepare 1 mM H with deionized water 2 o 2 , the concentration was calibrated by UV spectroscopy (H 2 o 2 for ε 240 nm=39.4m -1 cm -1 ). In addition, 100 mM 2,4,6-trichlorophenol (TCP) stock solution was prepared with 50% ethanol solution.
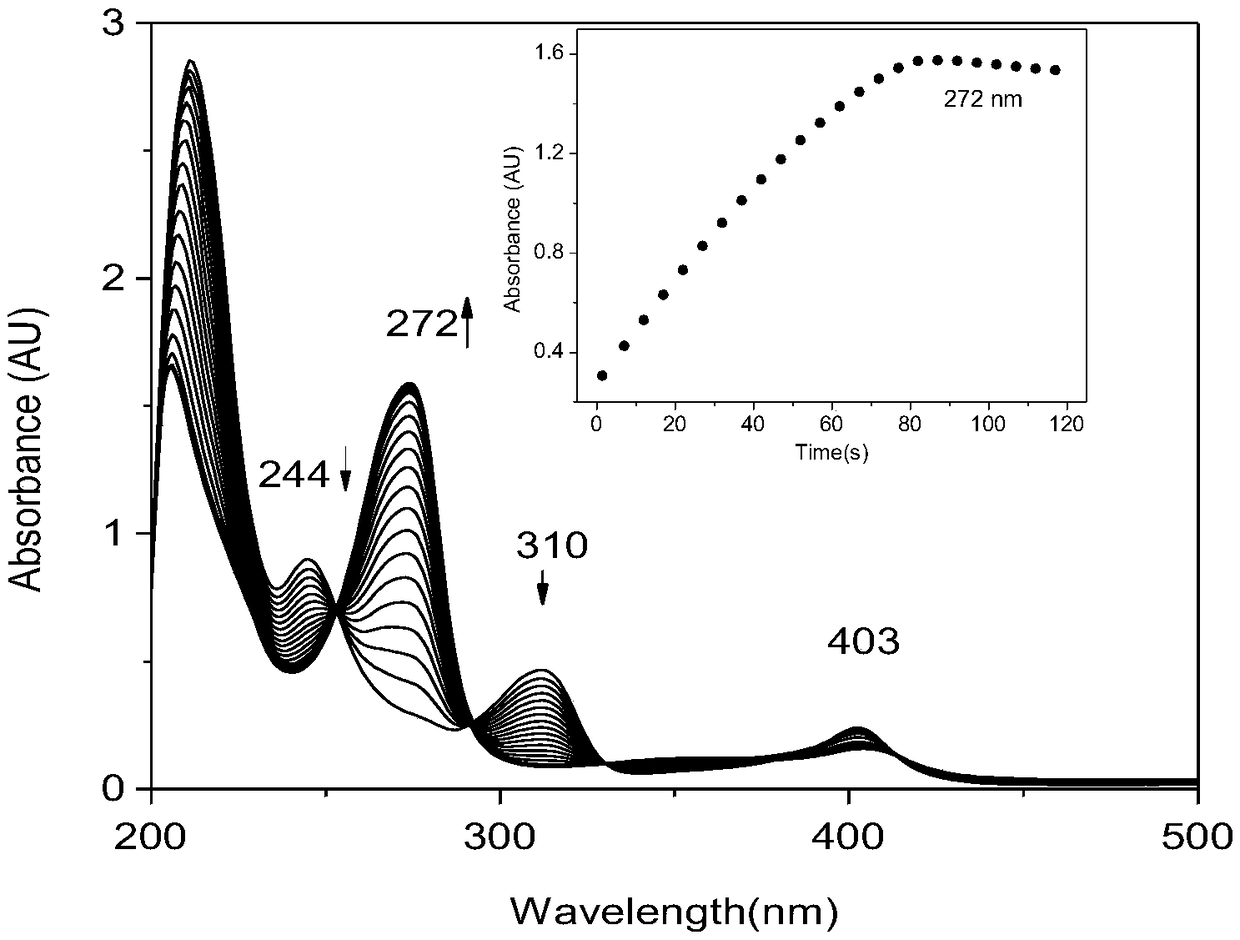
[0040] Take 1 mL of the above 4 μM F43Y / H64D Mb solution in a dry cuvette (1 cm), add 2 μL of 100 mM TCP to make the final concentration 0.1 mM, and then add 1 mL of 1 mM H 2 o 2 , start the reaction, react for 120s, collect a spectrum every 5s ( figure 2 ), where the absorbance of the product at 272nm varies with time see figure 2 illustration.
[0041] Depend on figure 2 It can be seen that the absorption peaks of the substrate TCP at 244nm and 311nm gradually disappeared, and the absorption peak of the product at 272nm gradually increased, and within 90 seconds, the substrate TCP was completely transformed into the product DCQ.
Embodiment 3
[0043] Prepare 2μM F43Y / H64D Mb and H64D Mb respectively with 100mM phosphate buffer (pH 7.0) (J.Xu, O.Shoji, T.Fujishiro, T.Ohki, T.Ueno and Y.Watanabe, Catal.Sci.Technol .,2012,2,739-744.), F43Y Mb (F.Liao,B.He,K.-J.Du,S.-Q.Gao,G.-B.Wen,Y.-W.Lin.ChemistryLetters , 2016, 45(9):1087-1089), WT Mb. At the same time, prepare 20 mM H with deionized water 2 o 2 , the concentration was calibrated by UV spectroscopy (H 2 o 2 for ε 240 nm=39.4m -1 cm -1 ), and prepare 10 mM 2,4,6-trichlorophenol (TCP) stock solution with 50% ethanol solution.
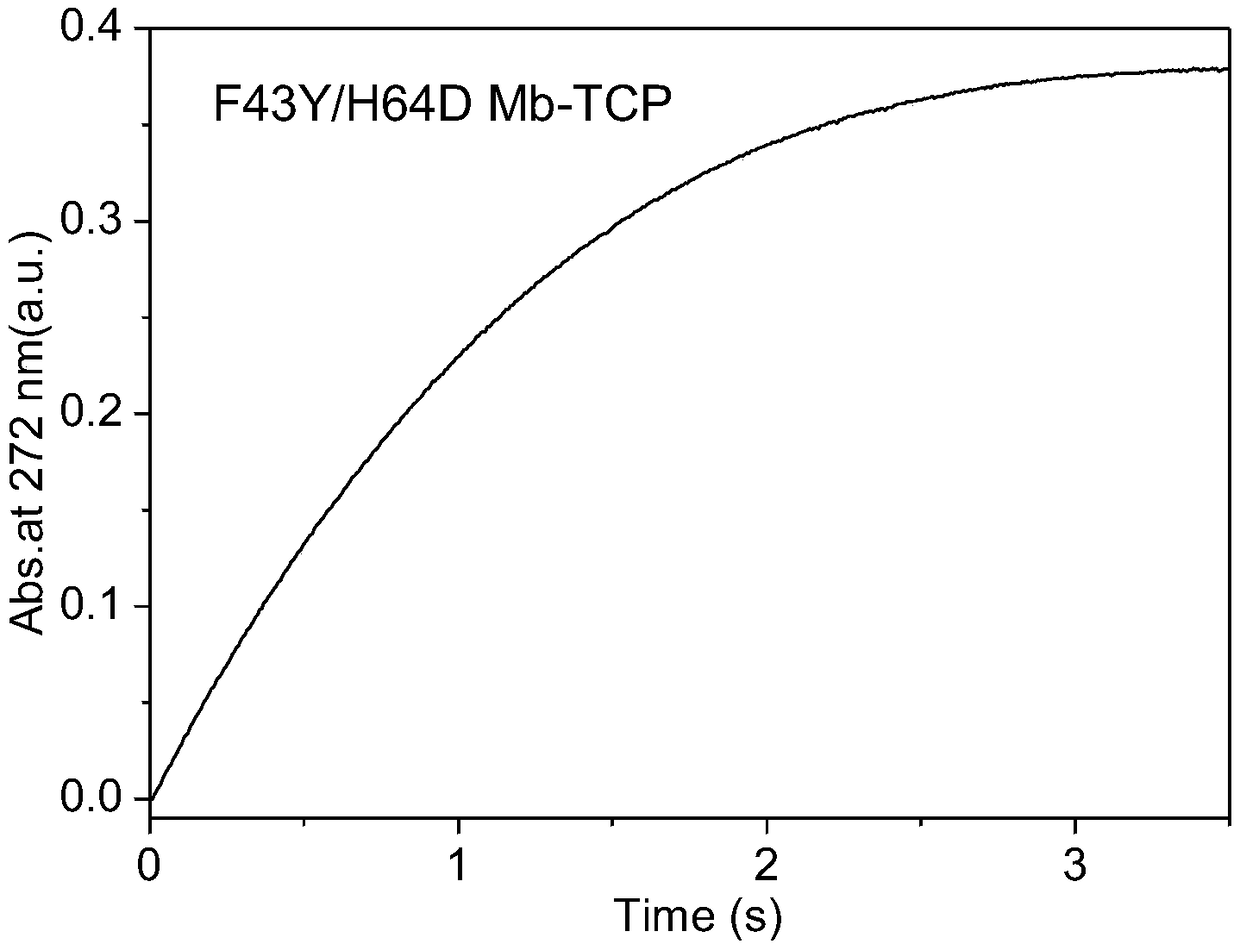
[0044] Take 2mL of the above three protein solutions respectively, add 12μL of 10mM TCP mother solution, mix evenly and place it in the injector of the rapid dwell spectrometer C, and take 20mM H 2 o 2 2mL was placed in the D injector. The catalytic reaction was carried out after injecting and mixing samples from C and D injectors, and the reaction time was 3.5s. The variation of the characteristic absorption peak at 272 nm of the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com