Nano-carrier for tanshinone IIA and application thereof
A nano-carrier and tanshinone technology, applied in the field of biomedicine, can solve problems such as lack of sustained release effect, increased number of administrations, and cytotoxicity, and achieve the effects of improving bioavailability, improving solubility, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
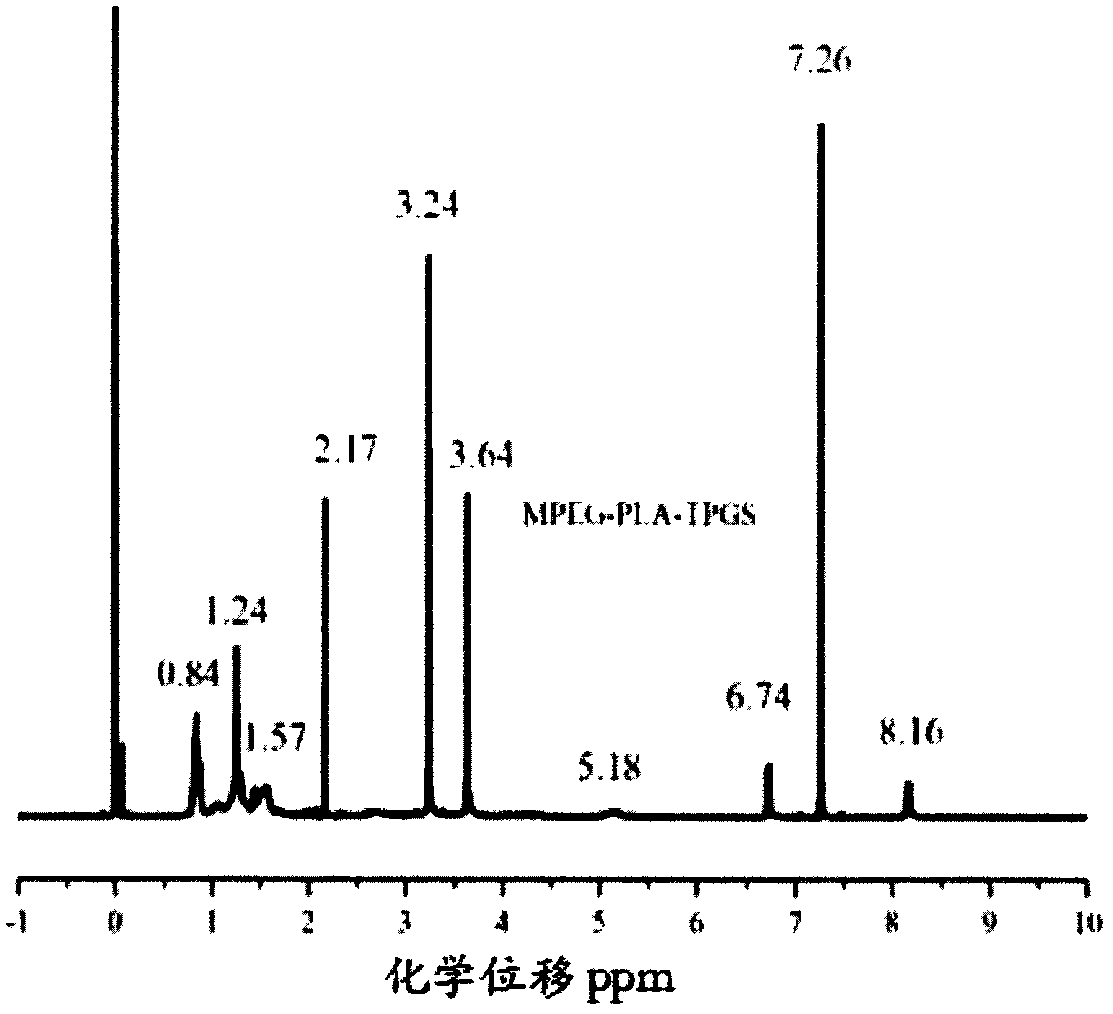
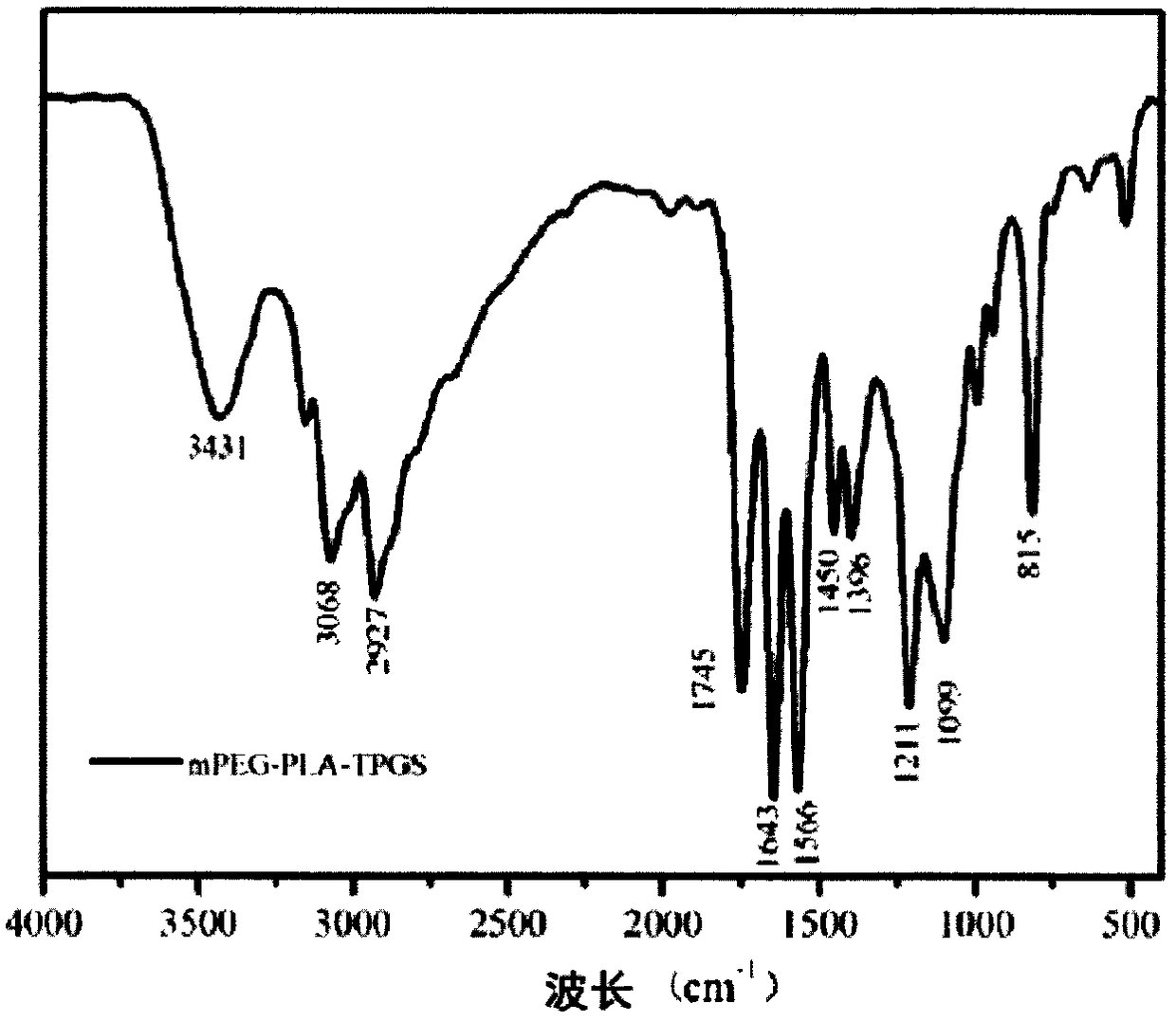
Image
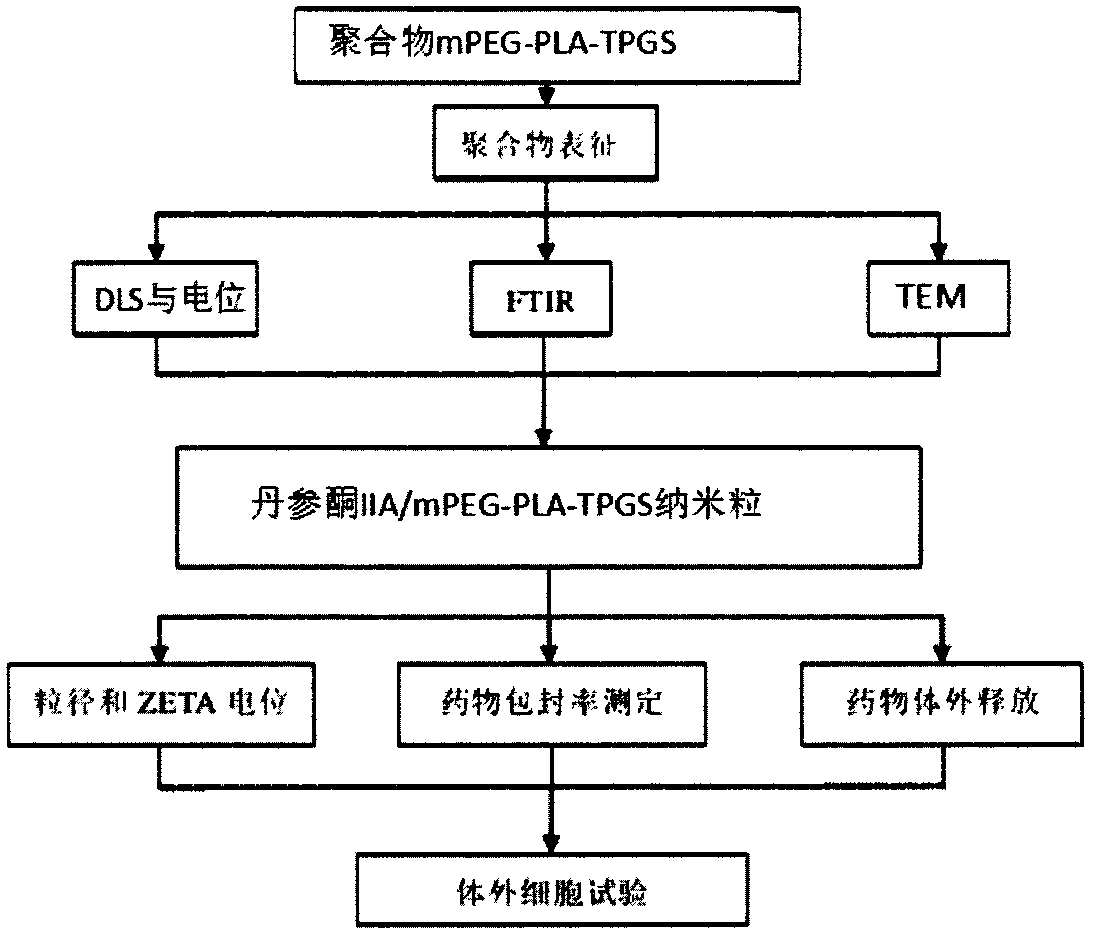
Examples
Embodiment 1
[0043] This embodiment provides a method for synthesizing nanocarriers, which includes the following steps in turn:
[0044] S1) Dissolve 1 part of L-lactide, 1 part of polyethylene glycol monoether and 0.1 part of stannous isooctanoate in dichloromethane, and react at 100°C for 48 hours; after the reaction, precipitate 3 times in glacial ether, and then vacuum drying at 40°C for 3 days to obtain mPEG-PLA-OH; in step S1), the solid-liquid ratio of the L-lactide to the methylene chloride is 0.72g / mL, and the mPEG-PLA-OH The structural formula is:
[0045]
[0046] Among them, y≥2;
[0047] S2) Dissolve 1 part of mPEG-PLA-OH, 1 part of succinic anhydride and 0.5 part of 4-dimethylaminopyridine in chloroform, mix well and add triethylamine; after reacting at room temperature for 3 days, precipitate in ether for 3 times, and filtered; after filtering, vacuum-dried at 40°C for 3 days to obtain mPEG-PLA-COOH; in step S2), the solid-liquid ratio of the mPEG-PLA-OH to the chlorof...
Embodiment 2
[0053] This embodiment provides a method for synthesizing nanocarriers, which includes the following steps in turn:
[0054] S1) Dissolve 14.4 parts of L-lactide, 7.6 parts of polyethylene glycol monoether and 0.2 part of stannous isooctanoate in dichloromethane, and react at 130°C for 18 hours; after the reaction, precipitate in glacial ether for 3 times, and then vacuum drying at 40°C for 3 days to obtain mPEG-PLA-OH; in step S1), the solid-liquid ratio of the L-lactide to the methylene chloride is 0.72 g / mL of the mPEG-PLA-OH The structural formula is:
[0055]
[0056] Among them, y≥2;
[0057] S2) Dissolve 10 parts of mPEG-PLA-OH, 2 parts of succinic anhydride and 1.2 parts of 4-dimethylaminopyridine in chloroform, mix well and add triethylamine; after reacting at room temperature for 3 days, precipitate in ether and filter ; After filtration, vacuum-dry at 40°C for 3 days to obtain mPEG-PLA-COOH; in step S2), the solid-liquid ratio of the mPEG-PLA-OH to the chlorofo...
Embodiment 3
[0063] This embodiment provides a method for synthesizing nanocarriers, which includes the following steps in turn:
[0064] S1) Dissolve 50 parts of L-lactide, 40 parts of polyethylene glycol monoether and 10 parts of stannous isooctanoate in dichloromethane, and react at 160°C for 2 hours; after the reaction, precipitate 3 times in ice ether, and then vacuum drying at 40°C for 3 days to obtain mPEG-PLA-OH; in step S1), the solid-liquid ratio of the L-lactide to the methylene chloride is 0.72 g / mL of the mPEG-PLA-OH The structural formula is:
[0065]
[0066] Among them, y≥2;
[0067] S2) Dissolve 30 parts of mPEG-PLA-OH, 10 parts of succinic anhydride and 10 parts of 4-dimethylaminopyridine in chloroform, mix well and add triethylamine; after reacting at room temperature for 3 days, precipitate in ether and filter ; After filtration, vacuum-dry at 40°C for 3 days to obtain mPEG-PLA-COOH; in step S2), the solid-liquid ratio of the mPEG-PLA-OH to the chloroform is 0.1 g / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com