Anthraquinonoimidazole nucleoside analog and synthetic method and application thereof
A technology of anthraquinone-imidazole ribavirin and its synthesis method, which is applied in the fields of chemistry and medicine, can solve the problems affecting and restricting the long-term and extensive use of anthracyclines, and achieve good application prospects and novel structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
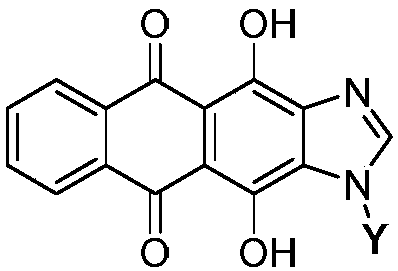
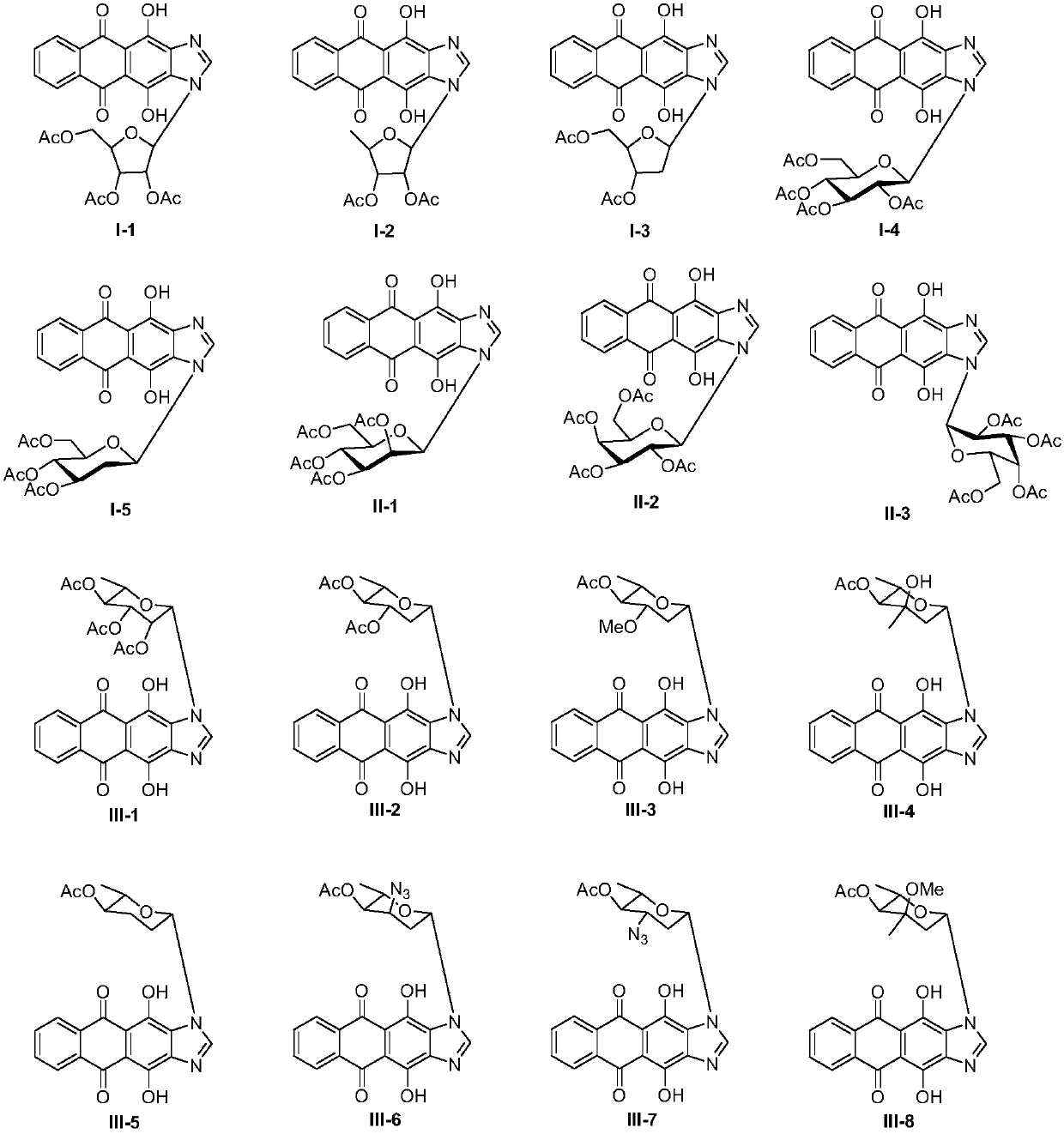
[0047] 1. Synthesis of glycosyl acceptor 4,11-dihydroxyanthra[2,3-d]imidazole-5,11-dione 5
[0048] Take 8g of compound 1, namely 1,4-dihydroxy-9,10-anthraquinone, in a 250mL round bottom flask, add 100mL of glacial acetic acid, stir and mix evenly, then add 5mL of concentrated nitric acid, heat and stir at 60°C for 3.5h. The reaction solution was filtered, and the filter cake was first washed with 15 mL of glacial acetic acid, and then washed with a large amount of water until neutral, and dried in vacuum to obtain compound 2 with a yield of 69%.
[0049] Take 2.5g of hydroxylamine hydrochloride in a 100mL round-bottom flask, add 80mL of absolute ethanol to dissolve, then add 1.5g of potassium hydroxide, stir for 30min, filter out the KCl white solid, take the filtrate and place it in a 250mL round-bottom flask, and put 2.5g Compound 2 was added to the above filtrate, stirred for 1.5 h, filtered, the filter cake was washed with absolute ethanol and water respectively, and dri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com