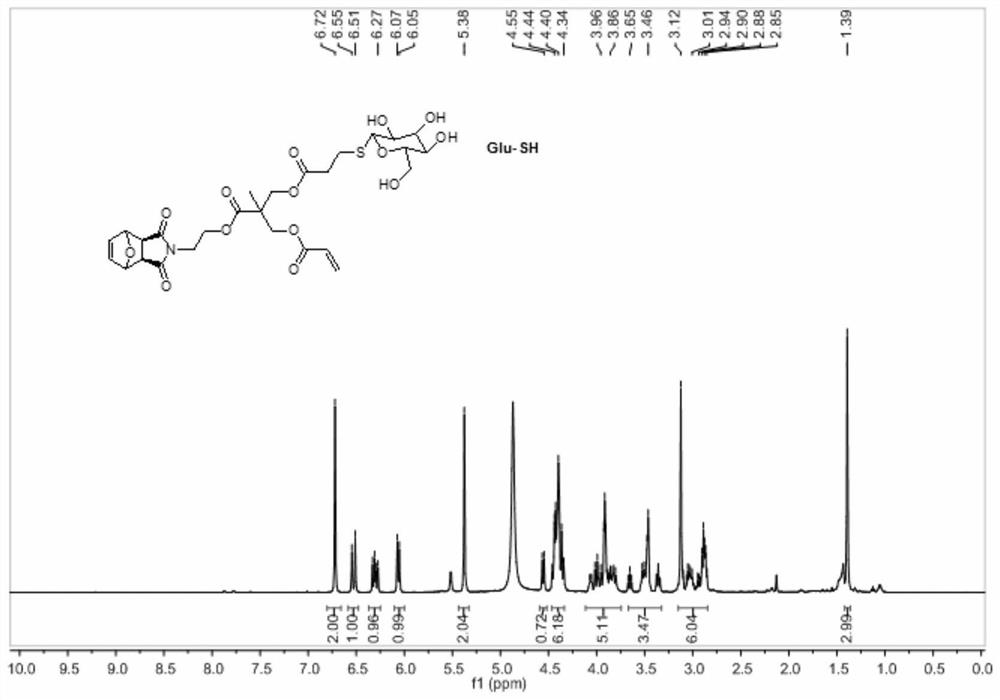
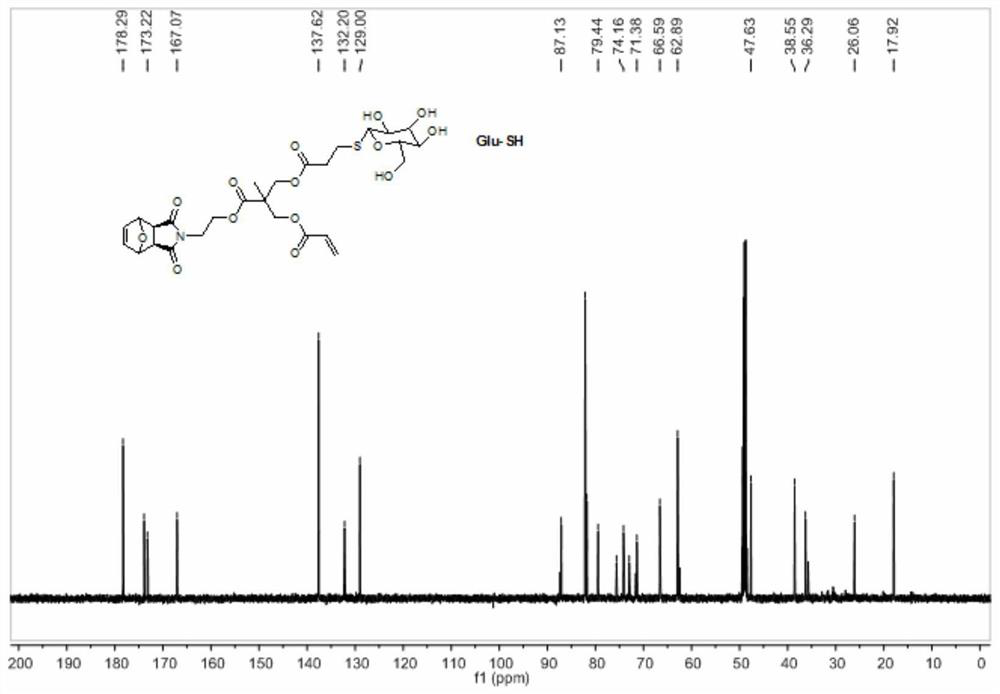
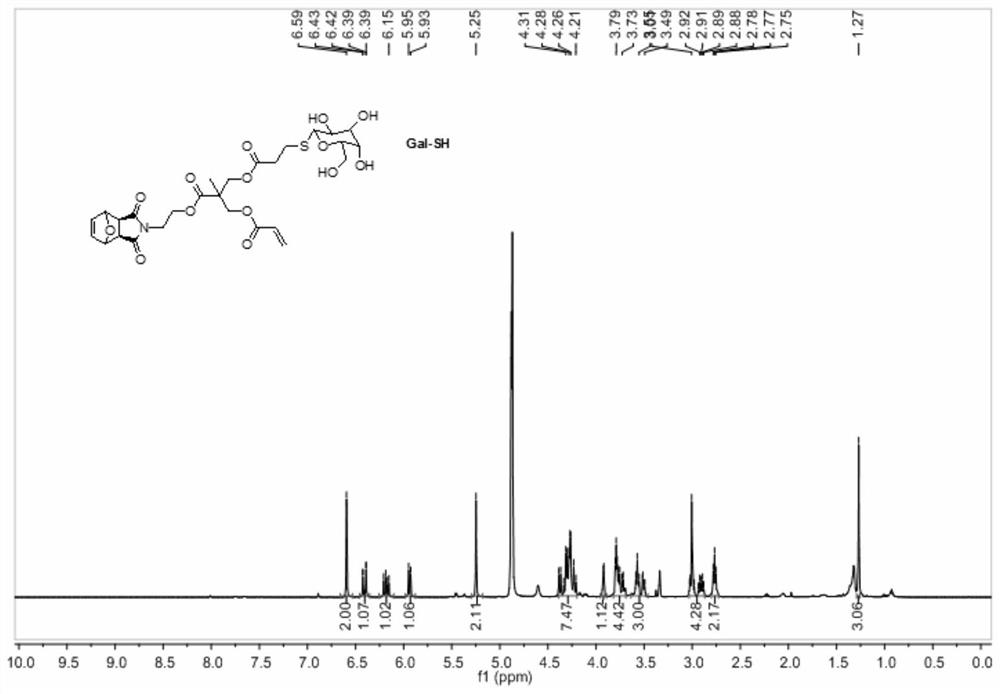
A method for preparing sugar-containing polymers by combining romp polymerization and mercapto-ene addition reaction
An addition reaction and polymer technology, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of complex multi-step operations of block copolymerization, and achieve regular structure, controllable structure, and synthetic method. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1. Synthesis of Precursor G1
[0049] Step 1) Exo-3,6-epoxy-1,2,3,6-tetrahydrophthalic anhydride was added into methanol and stirred evenly, cooled to 0°C in an ice-water bath and kept at a constant temperature. Dissolve ethanolamine in methanol, add slowly, dropwise for 0.5h, stir at low temperature for 15min, then react at room temperature for 0.5h, and finally reflux for 12h. After the reaction stopped, carry out rotary evaporation when cooling to room temperature, and use H 2 O and CH 2 Cl 2 Extracted and separated, and spin-dried to obtain a white solid 1;
[0050]Step 2) Dissolve 2,2,5-trimethyl-1,3-dioxane-5-carboxylic acid and 1 in dry dichloromethane, add N,N'-dicycloethylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP), stirred at room temperature for 24 h. After the reaction was stopped, the white solid was removed by filtration, and the filtrate was purified and separated by silica gel chromatography, and finally the white solid 2 was obtained;
...
Embodiment 2
[0071] In a 10 mL reaction vial, add heterogeneous sugar-containing monomer NB-Glu-Gal (40 mg, 0.048 mmol) and Grubbs3 rd The catalyst (2.3mg, 0.002mmol) was stuffed into a dense rubber stopper and sealed with a parafilm, and after passing through nitrogen for 10min, anhydrous N,N'-dimethylformamide (2mL) was passed through. After stirring and reacting at 65° C. for 24 h, EVE (0.1 mL) was added, stirred at room temperature for 30 min, and the reaction liquid was added dropwise to excess methanol for sedimentation. Centrifuge, remove the supernatant, and dry to obtain a tan polymer. The gel permeation chromatogram of PNB-Glu-Gal is shown in Figure 19 . Number average molecular weight Mn=10055, molecular weight M w / M n The distribution index was 1.18.
[0072] 9. Specific recognition of sugar-containing polymers and concanavalin A by nephelometric method
[0073] Prepare concanavalin A (1mg / mL) in HBS buffer solution (HEPES, 10mmol / L), pH=7.4, NaCl (50mmol / L), CaCl 2 (5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com