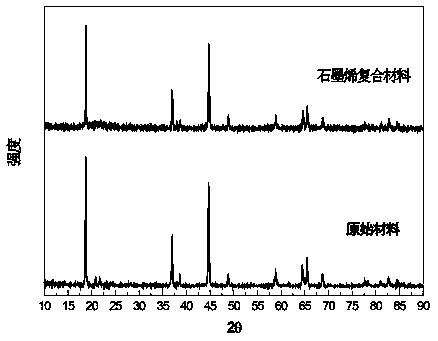
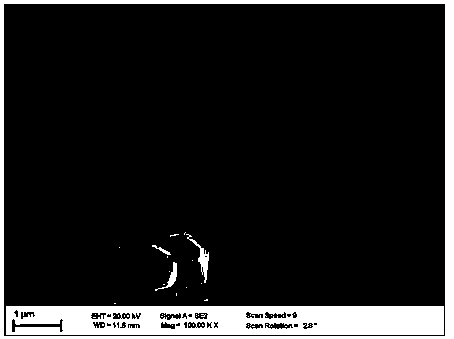
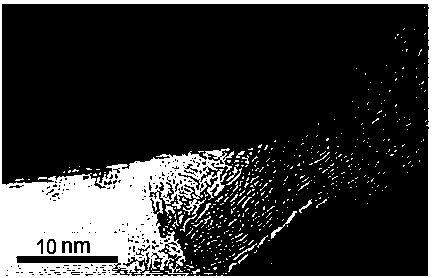
Method for preparing graphene composite lithium-rich positive electrode material through solvent-assisted reducing agent method
A lithium-rich cathode material and graphene composite technology, applied in the direction of positive electrodes, battery electrodes, active material electrodes, etc., can solve the problems of poor cycle stability and low first-cycle efficiency, and achieve reduced polarization, rate performance and cycle performance Enhanced and compounded effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In this example, glucose is used as the reducing agent, and the graphene composite cathode material prepared in ethanol solvent is 0.4Li2MnO3·0.4Li[Ni1 / 3Co1 / 3Mn1 / 3]O2 / GO.
[0023] Prepare 2mol / L Ni(CH3COO)2·4H2O, Co(CH3COO)2·4H2O and Mn(CH3COO)2·4H2O metal salt mixed solution A according to the stoichiometric ratio, prepare 4mol / L Na2CO3 and 1.5mol / L In a 5L reactor, A and B were mixed and co-precipitated, the pH of the reaction was controlled to be 9, the rotation speed was 800rpm / min, the temperature was 50 degrees, the reaction was 24h, and the stirring and aging was completed for 24h. Suction filter out the precipitate, dry it, weigh an excess of 5% Li2CO3 according to the metering ratio, mix and ball mill it, put it into a muffle furnace, raise the temperature from room temperature to 500°C in 3 hours, keep it warm for 5 hours, and then raise the temperature to 900°C in 5 hours. Keep it warm for 12 hours, then grind it for 20 minutes after cooling in the furnace to...
Embodiment 2
[0027] In this example, glucose is used as the reducing agent, and the graphene composite cathode material prepared in ethylene glycol solvent is 0.6Li2MnO3·0.1Li[Ni0.4Co0.2Mn0.2]O2 / GO.
[0028] Prepare 0.5mol / L Ni(CH3COO)2·4H2O, Co(CH3COO)2·4H2O and Mn(CH3COO)2·4H2O metal salt mixed solution A according to the stoichiometric ratio, prepare 1mol / L NaOH and 1.2mol / L Mix L of ammonia solution B, in a 1L beaker, mix and co-precipitate A and B, control the pH of the reaction to 11, rotate at 1000rpm / min, and temperature at 55 degrees, react for 4h, and finish stirring and aging for 24h. Suction filter out the precipitate, dry it, weigh an excess of 5% LiOH according to the metering ratio, mix and ball mill it, put it into a muffle furnace, raise the temperature from room temperature to 550°C in 3 hours, keep it warm for 5 hours, and then raise the temperature to 920°C in 5 hours. Keep it warm for 12 hours, then grind for 20 minutes after cooling in the furnace, and then obtain the...
Embodiment 3
[0032] In this example, ascorbic acid was used as the reducing agent, and the graphene composite cathode material prepared in NMP (nitrogen-methylpyrrolidone) solvent was 0.4Li2MnO3·0.4Li[Ni0.5Co0.2Mn0.3]O2 / GO.
[0033] Prepare 1mol / L Ni(CH3COO)2 4H2O, Co(CH3COO)2 4H2O and Mn(CH3COO)2 4H2O metal salt mixed solution A according to the stoichiometric ratio, prepare 2mol / L NaOH and 1.2mol / L In a 1L beaker, mix and co-precipitate A and B, control the pH of the reaction to 9, rotate at 900 rpm / min, and temperature at 50 degrees, react for 12 hours, and finish stirring and aging for 24 hours. Suction filter out the precipitate, dry it, weigh an excess of 5% Li2CO3 according to the metering ratio, mix and ball mill it, put it into a muffle furnace, raise the temperature from room temperature to 500°C in 3 hours, keep it warm for 5 hours, and then raise the temperature to 900°C in 5 hours. Keep it warm for 12 hours, then grind for 20 minutes after cooling in the furnace, and then obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
| Average discharge specific capacity | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com