Method for selectively recycling positive electrode materials for lithium ion batteries
A technology for lithium-ion batteries and cathode materials, which is applied in the field of selective recovery of cathode materials for lithium-ion batteries, and can solve problems such as low recovery rate, limited industrial application value, and cumbersome recycling procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
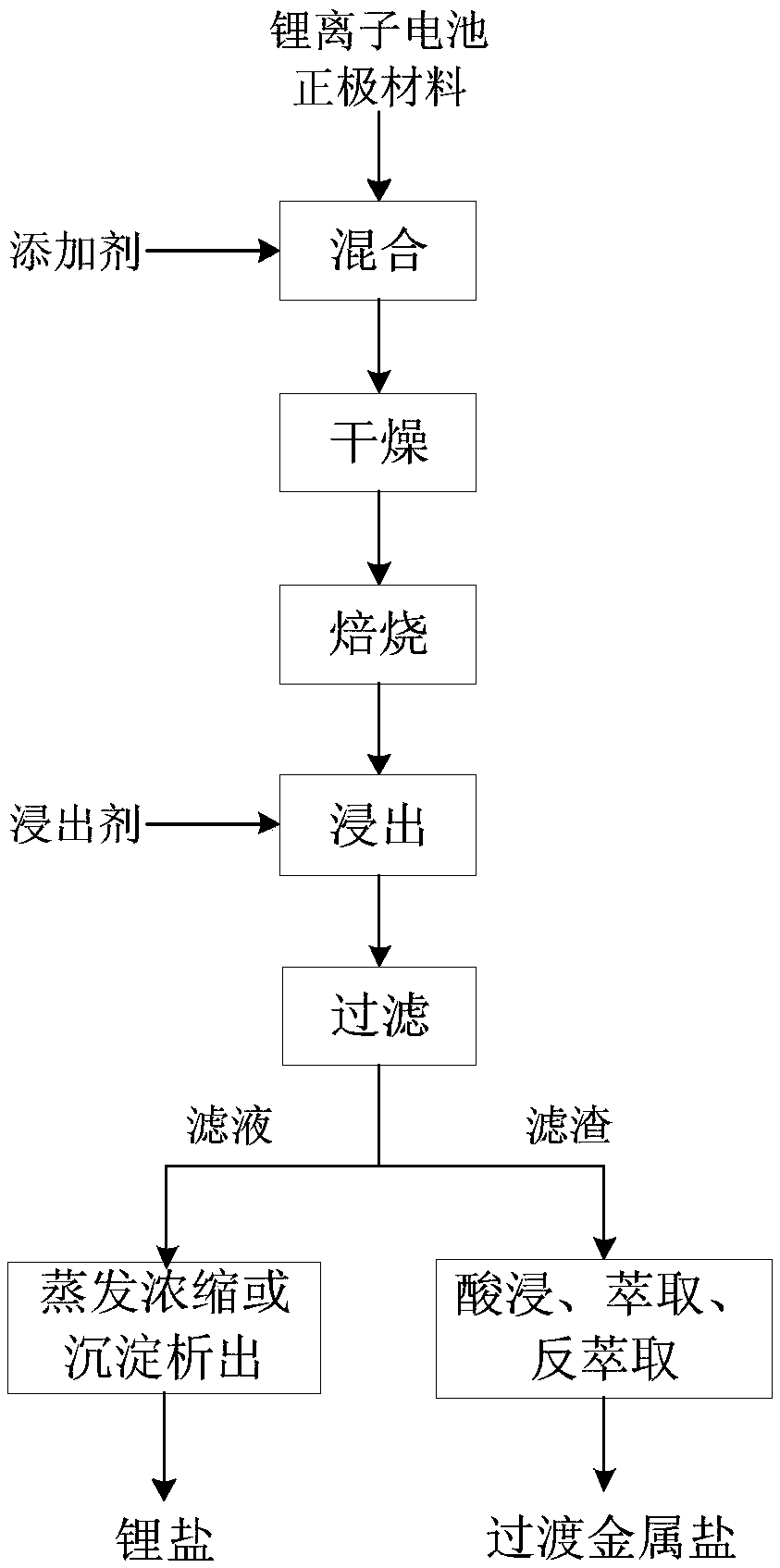
[0065] This embodiment provides a method for selectively recovering the positive electrode material of a lithium ion battery. The lithium ion battery is a waste lithium cobalt oxide battery, and the metal element composition of the positive electrode material is shown in Table 1.
[0066] Table 1 Composition of metal elements in cathode materials of waste lithium cobaltate batteries
[0067] Metal
Li
Al
Ni
co
mn
Content, wt%
6.81
0.52
0.03
59.23
0.01
[0068] The method comprises the steps of:
[0069] (1) After mixing the positive electrode material of lithium cobalt oxide battery with sulfuric acid at a molar ratio of 2:1, dry it at 150°C for 8 hours, and then bake it at 800°C for 2 hours. Carried out under vacuum conditions;
[0070] (2) leaching the roasted product obtained in step (1) with water, the solid-to-liquid ratio of the roasted product and water is 400g L -1 , leached at 20°C for 400 minutes, filtered and ...
Embodiment 2
[0074] This embodiment provides a method for selectively reclaiming the positive electrode material of a lithium-ion battery, and the lithium-ion battery is a waste nickel-cobalt lithium manganate battery (LiNi 0.5 co 0.2 mn 0.3 o 2 ), the metal element composition of its cathode material is shown in Table 2.
[0075] Table 2 Composition of metal elements in the positive electrode material of waste nickel-cobalt lithium manganese oxide battery
[0076] Metal
Li
Al
Ni
co
mn
Content, wt%
6.69
0.20
28.64
12.10
16.45
[0077] The method comprises the steps of:
[0078] (1) After mixing the positive electrode material of nickel-cobalt lithium manganese oxide battery, sulfuric acid and sodium sulfate in a molar ratio of 2:2:1, dry at 100°C for 12h, and then bake in air atmosphere at 500°C for 6h;
[0079] (2) leaching the roasted product obtained in step (1) with water, the solid-to-liquid ratio of the roasted product and w...
Embodiment 3
[0083] This embodiment provides a method for selectively reclaiming the positive electrode material of a lithium-ion battery, and the lithium-ion battery is a waste nickel-cobalt lithium manganate battery (LiNi 0.3 co 0.3 mn 0.3 o 2 ), the metal element composition of its cathode material is shown in Table 3.
[0084] Table 3 Composition of metal elements in the positive electrode material of waste nickel-cobalt lithium manganese oxide battery
[0085] Metal
Li
Al
Ni
co
mn
Content, wt%
6.71
0.19
20.64
20.10
18.45
[0086] The method comprises the steps of:
[0087] (1) After mixing the positive electrode material of nickel-cobalt lithium manganese oxide battery with sulfuric acid at a molar ratio of 5:1, dry it at 300°C for 0.2h, and then bake it at 1500°C for 0.5h in an argon atmosphere;
[0088] (2) leaching the roasted product obtained in step (1) with water, the solid-to-liquid ratio of the roasted product and wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com