Cavity type chelating agent and preparation method thereof
A chelating agent, crypt-shaped technology, applied in the field of homogeneous fluorescence immunoassay, can solve the problems of reducing the signal/noise ratio, reducing the analytical sensitivity, difficult to provide measurement signals, etc., to improve the sensitivity, reduce non-specific reactions, long fluorescence lifetime Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention also provides the preparation method of cryptate, this method comprises:
[0043] Step 1: Solvent, N,N',N,N'-[bis(2,2'-bipyridine-6,6'-dimethyl)]diamine, Li 2 CO 3 Add it into the reaction flask, reflux for 0.5-1.5h, then dropwise add the solution of 6,6'-dibromodimethyl-2,2'-bipyridyl-4-carboxylate methyl ester, and continue the reaction for 30-50h, Cooled to room temperature, the reaction solution was suction filtered, rotary evaporated, and separated by column chromatography to obtain the first intermediate;
[0044] The solvent is preferably acetonitrile, and the N,N',N,N'-[bis(2,2'-bipyridine-6,6'-dimethyl)]diamine and 6,6'- The molar ratio of methyl dibromodimethyl-2,2'-bipyridine-4-carboxylate is preferably 1:(1-2.5); more preferably 1:(1.5-2). The dripping time is preferably 1-2.5h; the specific reaction is as follows:
[0045]
[0046] Step 2: Add the solvent and the first intermediate obtained in Step 1 to the reaction flask, and t...
Embodiment 1
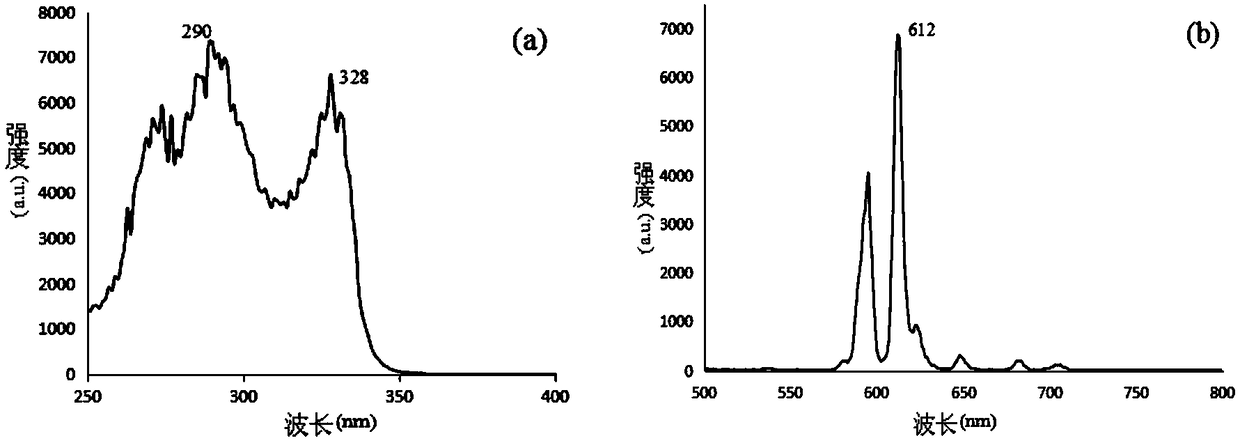
[0054] Embodiment 1 Preparation of chelating agent formula I
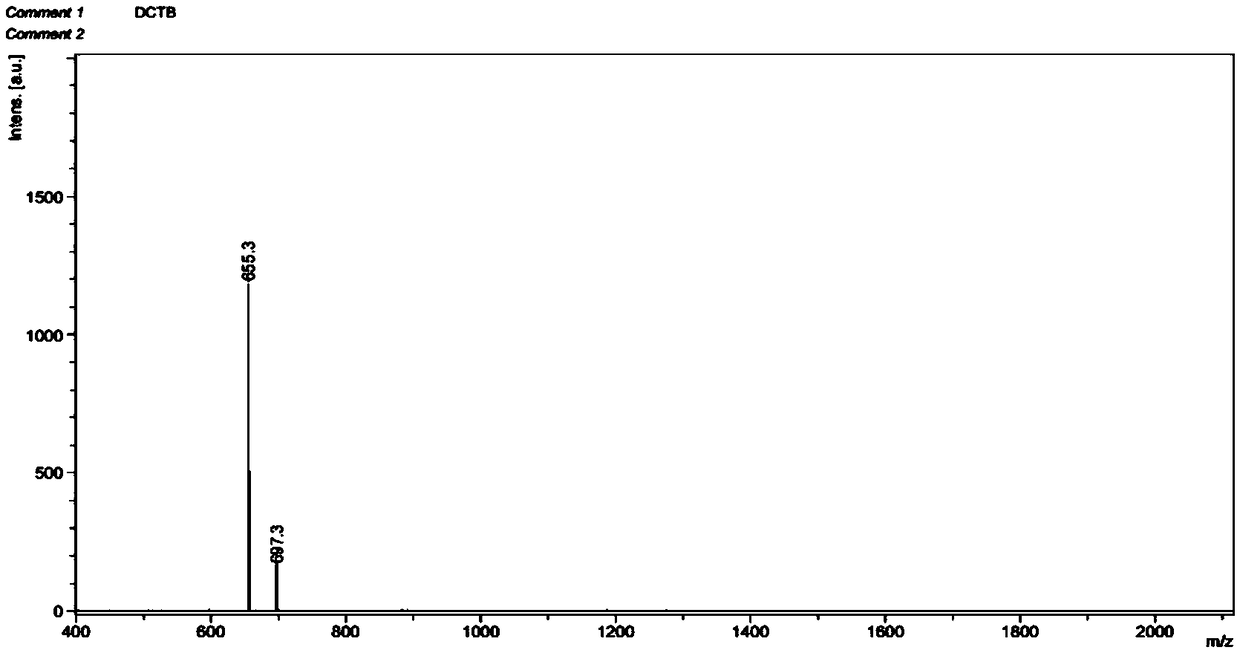
[0055] The compound N,N',N,N'-[bis(2,2'-bipyridine-6,6'-dimethyl)]diamine 0.338mmol and Li 2 CO 3 (3.387mmol) was dissolved in 267mL of acetonitrile, heated to reflux for 1h, slowly added dropwise 6,6'-dibromodimethyl-2,2'-bipyridine-4-carboxylic acid methyl ester (0.339mmol) within 1.0h 67mL of acetonitrile solution, reflux reaction for 30h, cooled to room temperature, the reaction solution was suction filtered, and rotary evaporated to obtain a crude product, which was separated by column chromatography to obtain the first intermediate C4 with a yield of 51.5%;
[0056] The first intermediate compound C4 (7.91 × 10 -5 mol), methanol 30mL, EuCl 3 (2.32×10 -4 mol) methanol solution was added to the reaction flask, stirred and refluxed for 23h, cooled to room temperature, and blown dry with nitrogen to obtain 47 mg of white precipitate, the second intermediate C5, which was dried for later use without purificati...
Embodiment 2
[0060] Embodiment 2 Preparation of chelating agent formula I
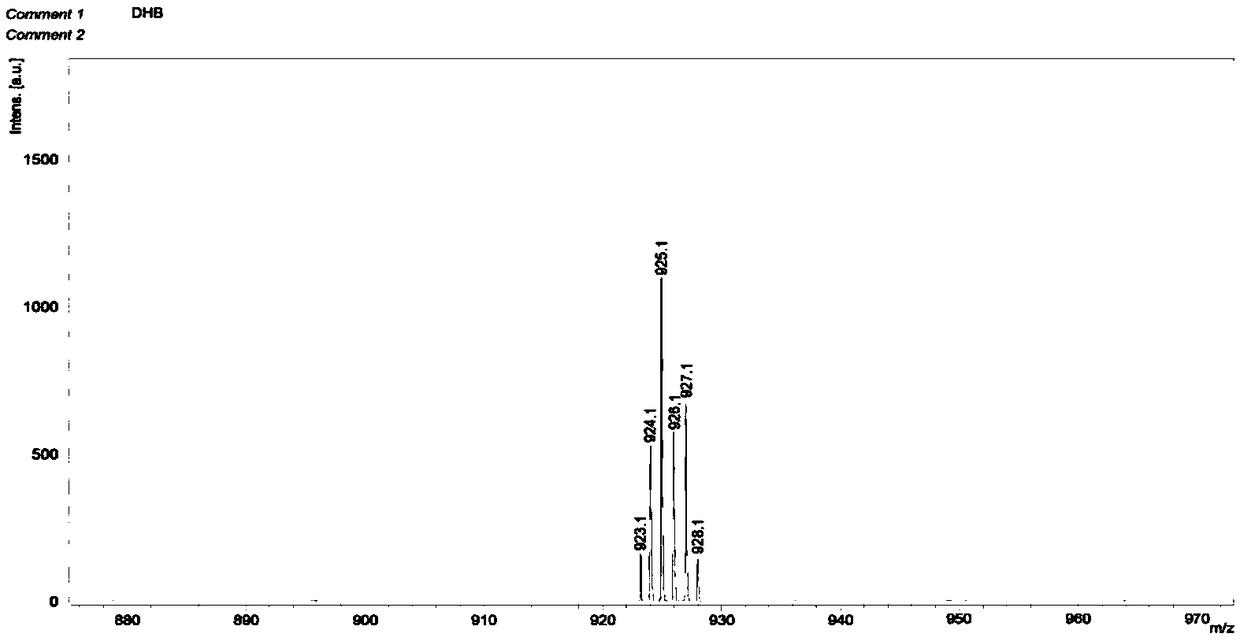
[0061] The compound N,N',N,N'-[bis(2,2'-bipyridine-6,6'-dimethyl)]diamine (0.338mmol) and Li 2 CO 3 (3.387mmol) acetonitrile solution, after heating up to reflux for 1h, slowly dropwise add 6,6'-dibromodimethyl-2,2'-bipyridine-4-carboxylic acid methyl ester (0.507mmol) in 1.0h Acetonitrile solution was reacted for 35 hours, cooled to room temperature, the reaction solution was suction filtered, and rotary evaporated to obtain a crude product, which was separated by column chromatography to obtain the first intermediate C4 with a yield of 53.9%.
[0062] The first intermediate compound C4 (7.91 × 10 -5 mol) with absolute methanol 30mL, EuCl 3 (1.58×10 -4 mol) methanol solution was added to the reaction flask, stirred and heated to reflux, continued to react for 23 h, cooled to room temperature, and blown dry the liquid in the cup with nitrogen to obtain 47 mg of the second intermediate C5 as a white precipitate....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com