Organic photovoltaic material as well as preparation method and application thereof
An organic photovoltaic material and reaction technology, applied in photovoltaic power generation, organic chemistry, semiconductor/solid-state device manufacturing, etc., can solve the problems of low efficiency of wide bandgap materials, ternary and laminated device preparation difficulties, etc., and achieve easy reaction process Control, easy adjustment of energy level and spectrum, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation process of IDDT-TBA:
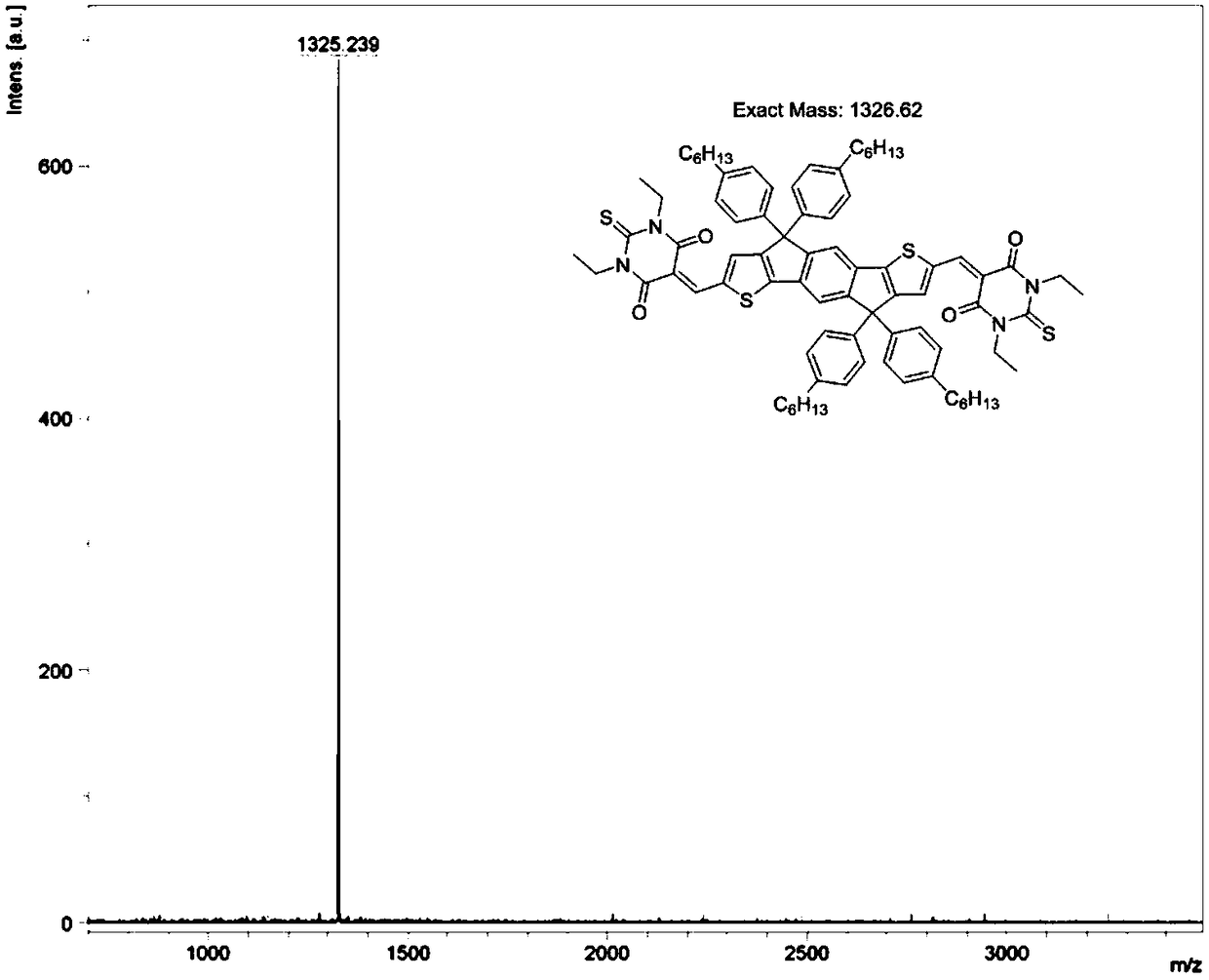
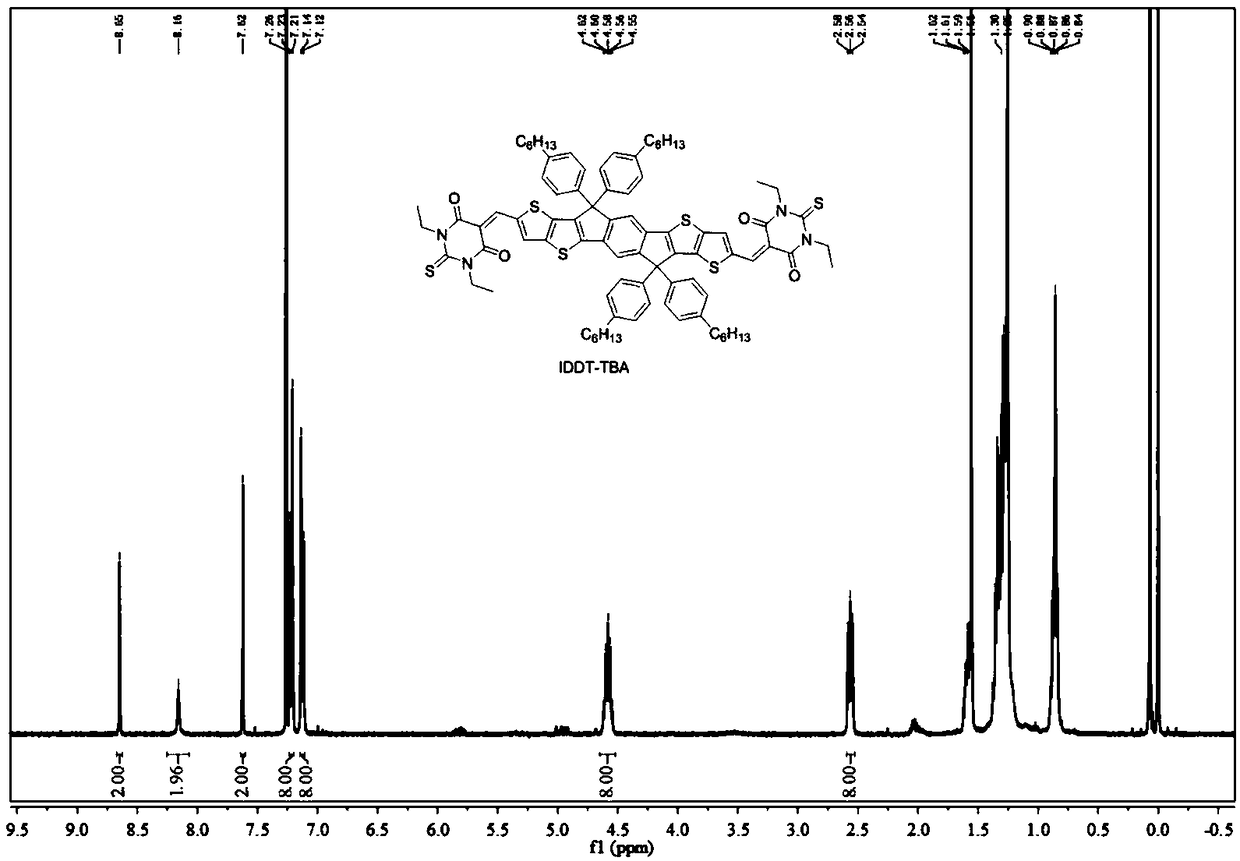
[0036] 6,6,12,12-Tetrakis(4-hexylphenyl)-6,12-dihydrodithieno[2,3-d:2',3'-d']-s-benzobisindeno [1,2-B:5,6-b']dithiophene-2,8-dicarbaldehyde (107.4 mg, 0.1 mmol) and TBA (44 mg, 0.22 mmol) were dissolved in 10 mL of chloroform, then 0.1 mL of piperidine was added . The solution was stirred at 80°C for 8 hours. The mixture was then diluted with chloroform and washed three times with brine. The organic phases were combined and washed with MgSO 4 dry. After removing the solvent, the resulting residue was purified by column chromatography using hexane as eluent to give IDDT-TBA (96.8 mg, 90.1%) as a dark blue solid. Implementation route:
[0037]
[0038] Such as image 3 as shown, 1 H NMR (400MHz, CDCl 3 ): δ8.65(s,2H), 8.16(s,2H), 7.62(s,2H), 7.22(d,8H), 7.13(d,8H), 4.58(m,8H), 2.56(m, 8H), 1.62-1.59 (m, 8H), 1.38-1.27 (m, 36H), 0.90-0.84 (m, 12H). Such as Figure 6 as shown, 13 C NMR (101MHz, CDCl 3 ):δ178.57,161.03,...
Embodiment 2
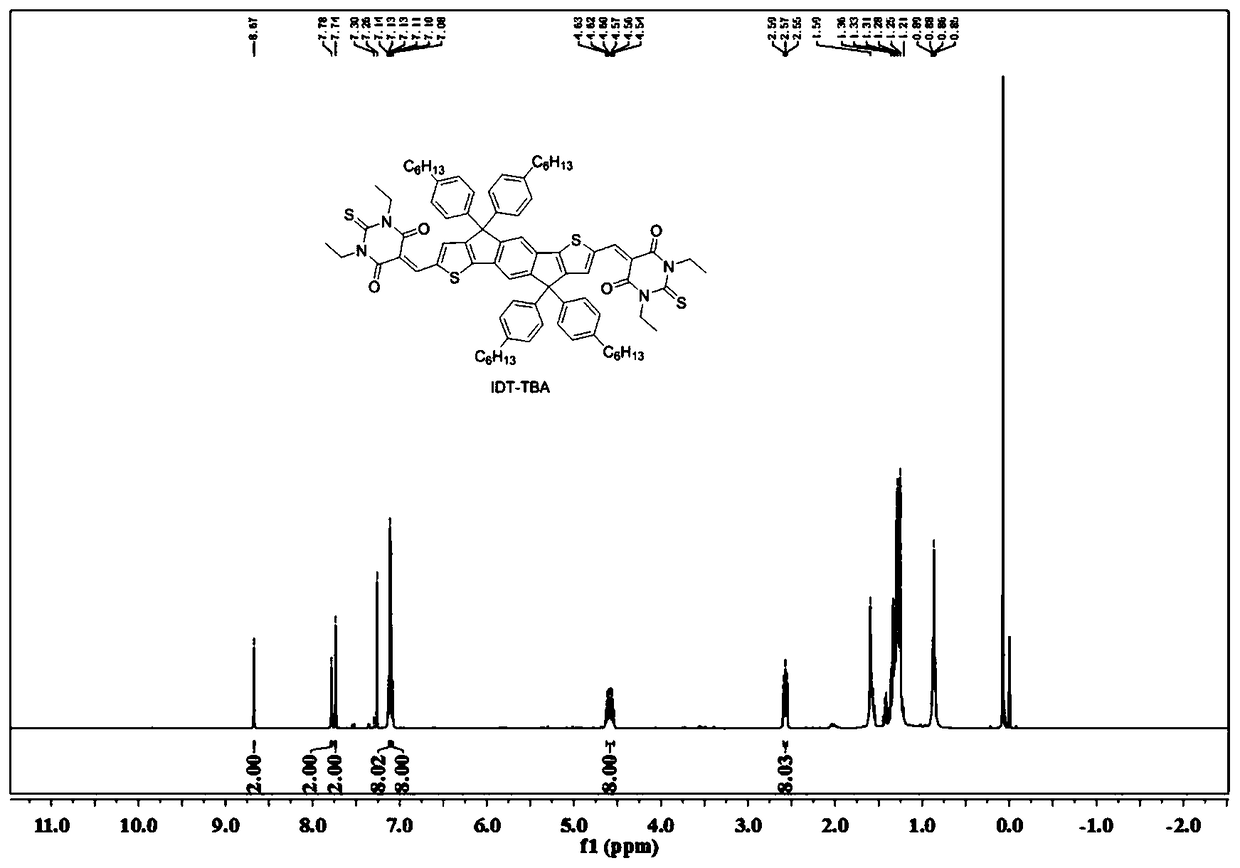
[0041] The preparation process of IDT-TBA: 4,4,9,9-tetra(4-hexylphenyl)-4,9-dihydro-s-benzobisindeno[1,2-B:5,6- B']dithiophene-2,7-dicarbaldehyde (96.25mg, 0.1mmol) and TBA (44mg, 0.22mmol) were placed in a two-necked reaction flask, and after adding 10mL of ethanol, slowly heated to 80°C until all the reactants were dissolved. After adding 0.1mL of triethylamine to ethanol, the solution turns red-blue. After the reaction, the product precipitates out of ethanol. After filtration, it is purified by column chromatography using hexane as the eluent to obtain IDT-TBA as a dark blue solid. (93.4 mg, 97%).
[0042] Implementation route:
[0043]
[0044] Such as figure 1 as shown, 1 H NMR (400MHz, CDCl 3 ): δ8.67(s,2H), 7.78(s,2H), 7.74(s,2H), 7.30(d,8H), 7.26(d,8H), 7.14-7.08(m,8H), 4.63- 4.54 (m, 8H), 2.59-2.55 (m, 8H), 1.59-1.21 (m, 36H), 0.89-0.85 (m, 12H). Such as Figure 5 as shown, 13 C NMR (101MHz, CDCl 3 ):δ178.51,160.86,160.05,159.77,157.67,156.12,149.76,142.3...
Embodiment 3
[0047] The preparation process of FTBr-TBA: the precursor 5,5'-(9,9-bis(6-bromohexyl)-9H-fluorene-2,7-diyl)bis(thiophene-2-carbaldehyde) (71.5mg ,0.1mmol) and TBA (44mg, 0.22mmol) were placed in a two-necked reaction flask, and after adding 10mL of ethanol, slowly heated to 80°C. Since the precursor had an alkyl chain bromide and had a high structural activity, there was no need to add it later Catalyzed by any base, the solution slowly turned blue-purple after 8 hours of reaction. After the reaction was completed, the column chromatography using hexane as the eluent was purified to obtain FTC8-TBA as a dark blue solid.
[0048] Implementation route:
[0049]
[0050] Such as Figure 4 as shown, 1 H NMR (400MHz, CDCl 3 )δ8.72(s,2H),7.96-7.95(d,2H),7.88(d,2H),7.83-7.79(m,4H),7.67-7.66(m,2H),4.70-4.60(m, 8H), 2.16-2.12(m,4H).
[0051] Figure 7 It is the thermal weight loss curve of IDDT-TBA. It can be seen that the material will reach the glass transition temperature w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com