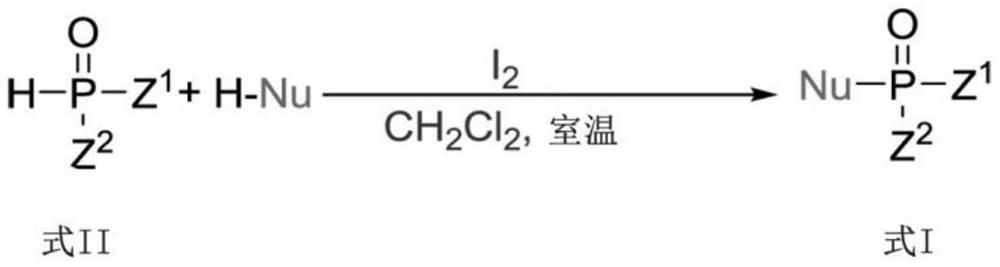
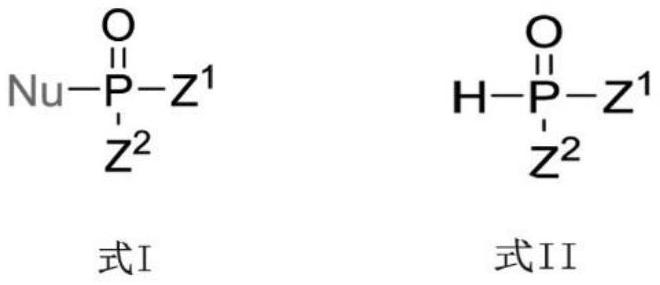Photoinduced Iodine Catalyzed Atherton-Todd Reaction Synthesis of Organophosphorus Compounds
A compound and light-induced technology, applied in the direction of organic chemistry, chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, etc., can solve problems such as limited development, environmental damage, single catalytic substrate, etc., and achieve high yield High, good universality, short response time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
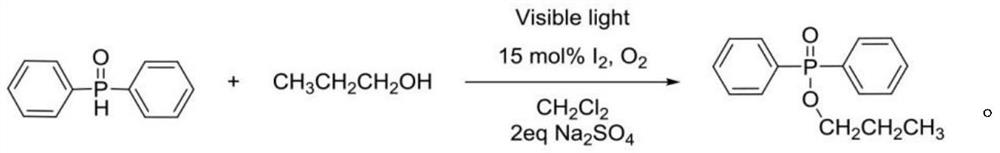
[0019] The preparation of embodiment one propyl diphenylphosphonate
[0020] Diphenylphosphine oxide (0.5mmol, 101mg), n-propanol (2.5mmol, 187ml), iodine (0.075mmol, 19mg), dichloromethane (3ml) were reacted under light at room temperature for 24h in an oxygen atmosphere, and 0.5ml was added Saturated sodium thiosulfate solution washes off iodine molecules, and after 2eq Na 2 SO 4 After drying, filtering, the organic phase was separated by column chromatography, and purified to obtain 104 mg of a white solid product with a yield of 80%. The reaction equation is as follows:
[0021]
[0022] The NMR characterization of propyl diphenylphosphonate is as follows: 1 H NMR (400MHz, CDCl 3 )δ7.85–7.77(m,4H),7.53–7.48(m,2H),7.47–7.41(m,4H),3.98(m,2H),1.75(m,J=14.1,7.1Hz,2H) , 0.97 (t, J=7.4Hz, 3H).
Embodiment 2
[0023] The preparation of embodiment two diphenyl phosphonic acid octyl esters
[0024] Diphenylphosphine oxide (0.5mmol, 101mg), n-octanol (2.5mmol, 392ml), iodine (0.075mmol, 19mg), dichloromethane (3ml) were reacted under light at room temperature for 24h in an oxygen atmosphere, and 0.5ml was added Saturated sodium thiosulfate solution washes off iodine molecules, and after 2eq Na 2 SO 4 After drying, filtering, the organic phase was separated by column chromatography, and purified to obtain 126 mg of light yellow oily liquid with a yield of 76%. The reaction equation is as follows:
[0025]
[0026] The NMR characterization of octyl diphenylphosphonate is as follows: 1 H NMR (400MHz, DMSO) δ7.80–7.70(m,4H),7.62–7.47(m,6H),3.97–3.86(m,2H),1.69–1.56(m,2H),1.27(d,J =46.0Hz, 10H), 0.86–0.79(m, 3H).
Embodiment 3
[0027] The preparation of embodiment three diphenylphosphonic acid cyclohexyl esters
[0028] Diphenylphosphine oxide (0.5mmol, 101mg), cyclohexanol (2.5mmol, 260ml), iodine (0.075mmol, 19mg), dichloromethane (3ml), light reaction for 24h in an oxygen atmosphere at room temperature, add 0.5ml Saturated sodium thiosulfate solution washes off iodine molecules, and after 2eq Na 2 SO 4 Drying, filtration, column separation of the organic phase, and purification gave 98 mg of a white solid product with a yield of 65%. The reaction equation is as follows:
[0029]
[0030] The NMR characterization of cyclohexyl diphenylphosphonate is as follows: 1 H NMR (400MHz, CDCl 3 )δ7.78–7.70(m,4H),7.45–7.39(m,2H),7.38–7.32(m,4H),4.40–4.29(m,1H),1.82(d,J=10.3Hz,2H) ,1.71–1.63(m,2H),1.58–1.48(m,2H),1.43–1.36(m,1H),1.23–1.15(m,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com