Synthesis method of cordycepin
A synthesis method and a technology for cordycepin, which are applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of expensive raw materials, increased manufacturing costs, etc., and achieve high yield, simple operation and little waste. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
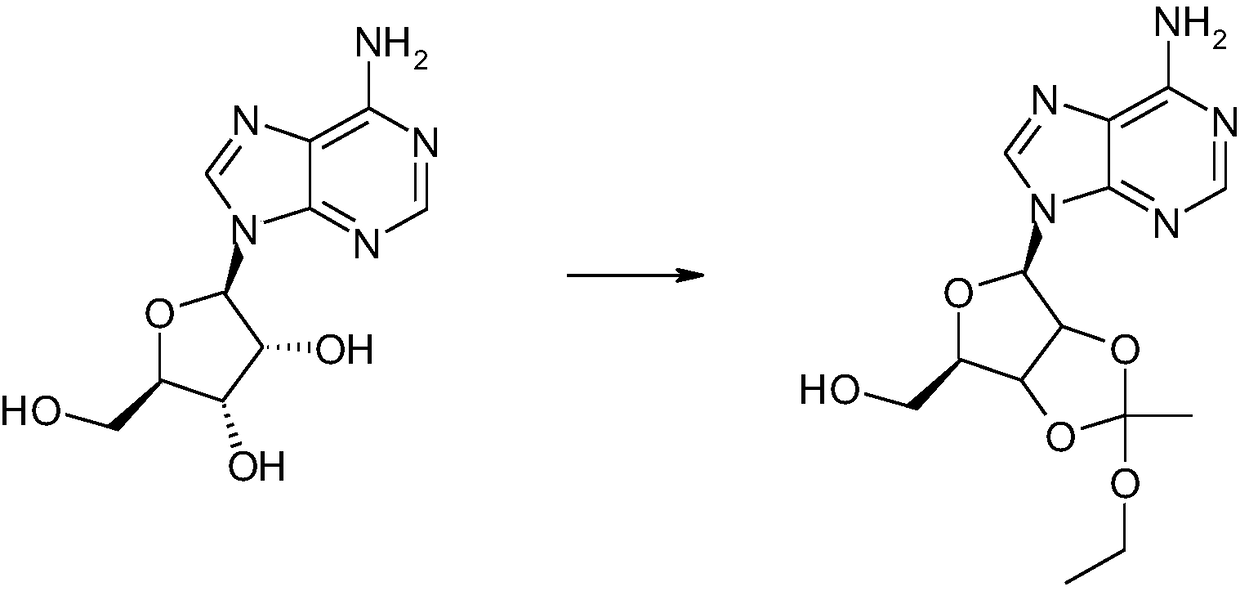
[0033] Put 2.35g of 9-β-D-ribofuranosyl adenine, 2g of triethyl orthoformate, and 12ml of anhydrous acetic acid into a 50ml reaction bottle, keep the reaction at 50°C for 3 hours, reduce the pressure to -0.08MPa, and concentrate to dryness. Get caramel A3.0g;
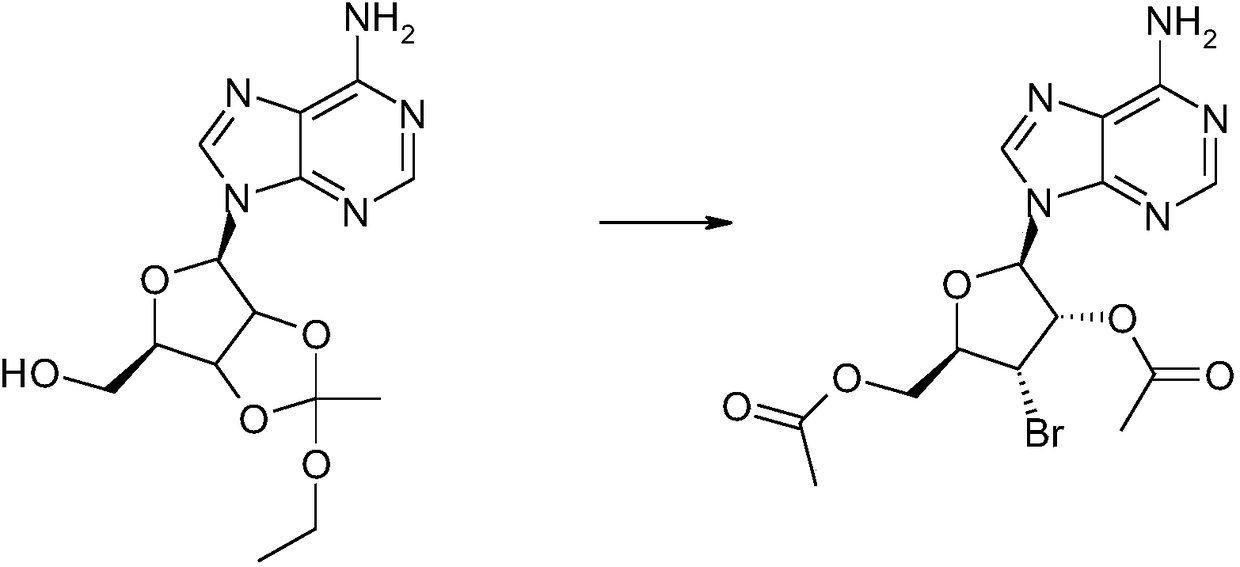
[0034] Dissolve 3.0 g of the caramel A obtained in the previous step in 17 ml of dichloromethane, add 6.5 g of acetyl bromide dropwise at 0°C, and react at room temperature for 5 hours. Slowly add 10ml of water dropwise at room temperature, after the drop is complete, stir at room temperature for 10 minutes, adjust the pH value to 8 with saturated sodium bicarbonate, separate the organic layer, and pass through a silica gel column with a mass ratio of dichloromethane:methanol=9:1 as the eluent Purified by chromatography to obtain caramel B1.9g;
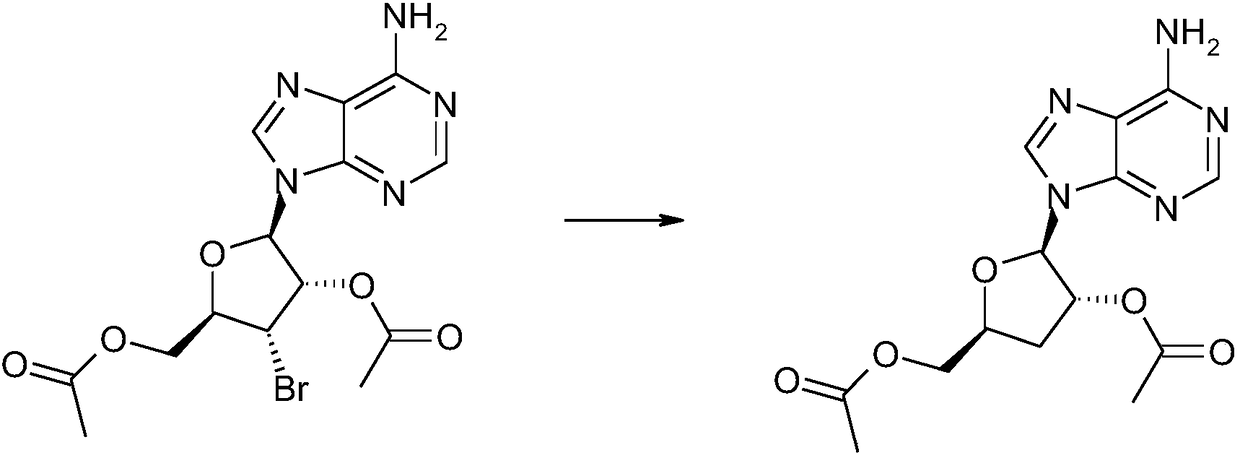
[0035] Add 20ml of triethylsilane and 10ml of trifluoroacetic acid to 1.9g of the obtained caramel B, heat to reflux for 12 hours, reduce the pressure to -0.08MPa, and concent...
Embodiment 2
[0037] Put 2g of 9-β-D-ribofuranosyl adenine, 1.71g of triethyl orthoformate, and 10ml of anhydrous acetic acid into a 50ml reaction bottle, keep the reaction at 50°C for 4 hours, reduce the pressure to -0.08MPa, and concentrate to dryness to obtain Caramel A2.6g;
[0038] Dissolve 2.6 g of caramel A obtained in the previous step in 16 ml of dichloromethane, add 6 g of acetyl bromide dropwise at 0°C, and react at room temperature for 5 hours after the drop is complete. Slowly add 10ml of water dropwise at room temperature, after the drop is complete, stir at room temperature for 10 minutes, adjust the pH value to 8 with saturated sodium bicarbonate, separate the organic layer, and pass through a silica gel column with a mass ratio of dichloromethane:methanol=9:1 as the eluent Purified by chromatography to obtain caramel B1.6g;
[0039] Add 21ml of triethylsilane and 8ml of trifluoroacetic acid to 1.6g of the obtained caramel B in the upward step, heat and reflux for 12 hours,...
Embodiment 3
[0041] Put 3.1g of 9-β-D-ribofuranosyl adenine, 2.5g of triethyl orthoformate, and 15ml of anhydrous acetic acid into a 50ml reaction bottle, keep the reaction at 50°C for 2 hours, reduce the pressure to -0.08MPa, and concentrate to dryness. Get caramel A3.4g;
[0042] Dissolve 3.4g of the caramel A obtained in the previous step in 18ml of dichloromethane, add 5.9g of acetyl bromide dropwise at 0°C, and react at room temperature for 6 hours. Slowly add 13ml of water dropwise at room temperature, after dropping, stir at room temperature for 10 minutes , the pH value was adjusted to 8 with saturated sodium bicarbonate, the organic layer was separated, and the mass ratio of dichloromethane:methanol=9:1 was used as the eluent to purify by silica gel column chromatography to obtain caramel B2.7g;
[0043] Add 25ml of triethylsilane and 12ml of trifluoroacetic acid to 2.7g of the obtained caramel B, heat to reflux for 12 hours, reduce the pressure to -0.08MPa, and concentrate to dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com