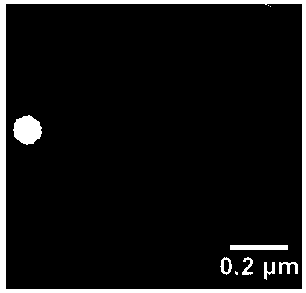
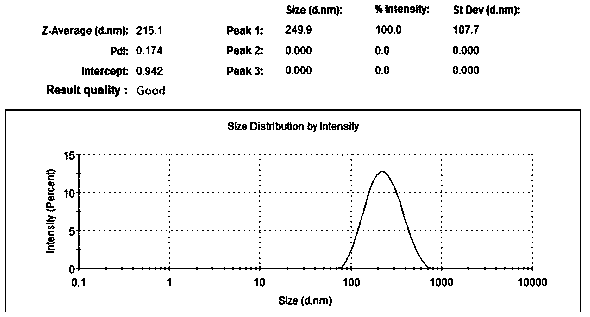
Fat-reducing drug resveratrol-loading controlled release nano carrier for oral administration and preparation method thereof
A nano-carrier, resveratrol technology, applied in the direction of drug combination, pharmaceutical formula, non-active ingredient medical preparations, etc., can solve the problems of low bioavailability, short half-life, poor water solubility of resveratrol, etc. Improves solubility, prevents accumulation of triglycerides, improves solubility and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Preparation of nanocarriers
[0035] 1. Dissolve 50 mg of PLGA in 5 ml of a mixed solvent of dichloromethane and acetone (volume ratio dichloromethane: acetone = 1:3) to form a uniform PLGA solution.
[0036] 2. Add 10 mg of free t-Res to the PLGA solution obtained in step 1, with an ultrasonic power of 100w and an ultrasonic time of 2min, and perform ice bath ultrasonication to obtain the s / o primary emulsion, which is the oil phase.
[0037] 3. Dissolve BSA in an appropriate amount of deionized water to obtain a uniform BSA solution with a concentration of 1%. This is the water phase. Add the oil phase obtained in step 2 dropwise to the water phase, and then perform ice bath ultrasound. The ultrasound power is 200w, ultrasonic time is 5min, to produce the final s / o / w emulsion.
[0038] 4. In order to disperse the final s / o / w emulsion, add 30ml of deionized water and stir with a magnetic force for 5h to remove the residual organic solvents to obtain a nanocarrier solution. ...
Embodiment 2
[0060] 1. Dissolve 100mg PLGA in 10ml of a mixed solvent of dichloromethane and acetone (volume ratio dichloromethane: acetone = 2:3) to form a uniform PLGA solution.
[0061] 2. Add 18 mg of free t-Res to the PLGA solution obtained in step 1, with an ultrasonic power of 200w and an ultrasonic time of 4min, and perform ice bath ultrasonication to obtain the s / o primary emulsion, which is the oil phase.
[0062] 3. Dissolve BSA in an appropriate amount of deionized water to obtain a uniform BSA solution with a concentration of 1%. This is the water phase. Add the oil phase obtained in step 2 dropwise to the water phase, and then perform ice bath ultrasound. The ultrasound power is 400w, ultrasonic time is 5min, to produce the final s / o / w emulsion.
[0063] 4. In order to disperse the final s / o / w emulsion, add 30ml of deionized water and stir with a magnetic force for 8h to remove the residual organic solvents to obtain a nanocarrier solution.
[0064] 5. Centrifuge the solution obtaine...
Embodiment 3
[0066] 1. Dissolve 80mg PLGA in 8ml of mixed solvent of dichloromethane and acetone (volume dichloromethane: acetone = 1:1) to form a uniform PLGA solution.
[0067] 2. Add 15mg of free t-Res to the PLGA solution obtained in step 1, with an ultrasonic power of 200w and an ultrasonic time of 3min, and perform ice bath ultrasonication to obtain the s / o primary emulsion, which is the oil phase.
[0068] 3. Dissolve BSA in an appropriate amount of deionized water to obtain a uniform BSA solution with a concentration of 1%. This is the water phase. Add the oil phase obtained in step 2 dropwise to the water phase, and then perform ice bath ultrasound. The ultrasound power is 400w, ultrasonic time is 5min, to produce the final s / o / w emulsion.
[0069] 4. In order to disperse the final s / o / w emulsion, add 30ml of deionized water and stir with a magnetic force for 6h to remove the residual organic solvents to obtain a nanocarrier solution.
[0070] 5. Centrifuge the solution obtained in step 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com