A polyunsaturated carboxylic acid modified metal organic framework material and its preparation method and application
A metal-organic framework, unsaturated technology, applied in chemical instruments and methods, other chemical processes, water/sludge/sewage treatment, etc., can solve the problem of low adsorption capacity, achieve high adsorption performance, short treatment cycle, and easy operation concise effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
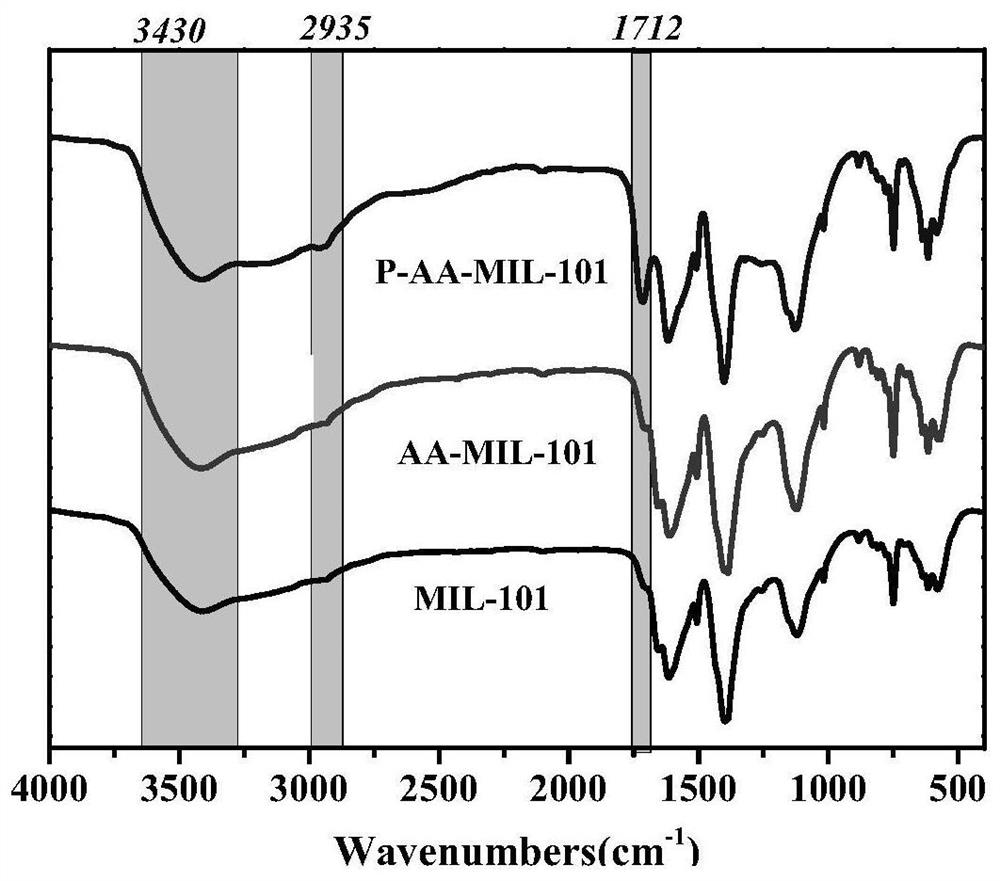
[0035] Example 1 Polyacrylic acid modified metal organic framework material P-AA-MIL-101
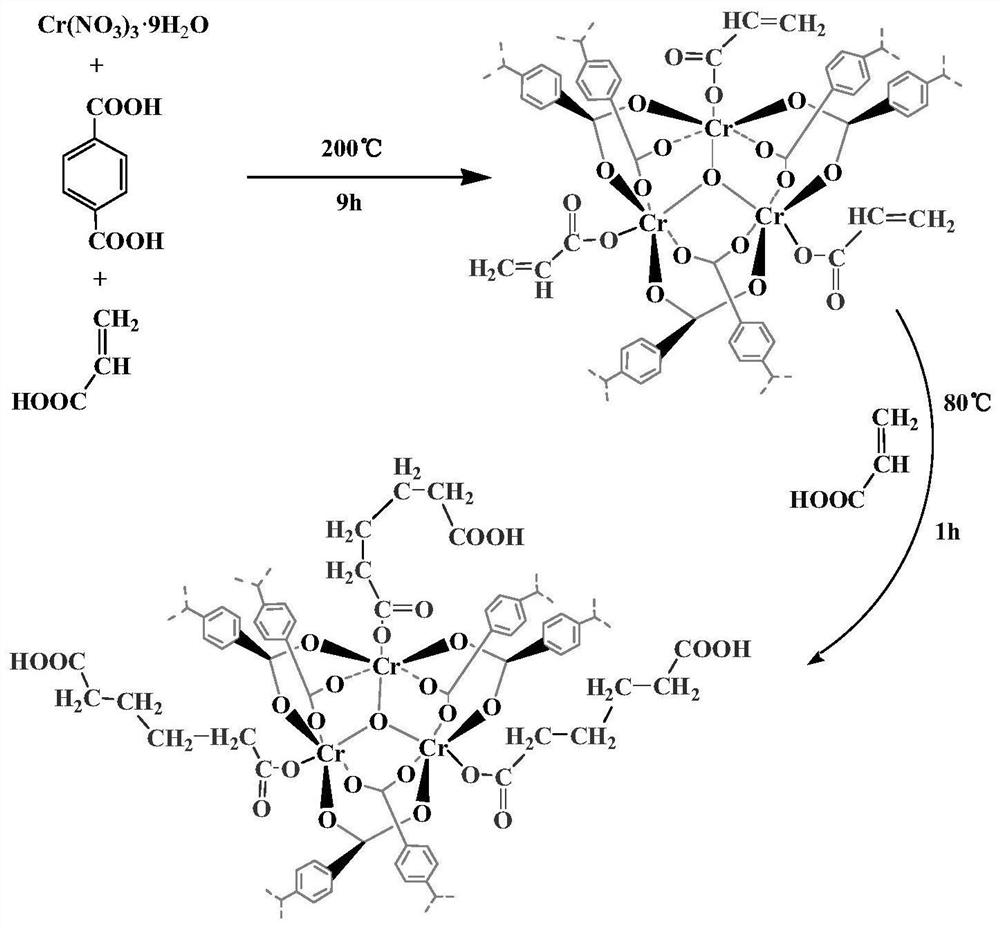
[0036] (1) The preparation method is as follows: the synthesis process is as follows figure 1 shown
[0037] 1) Weigh 3.2g of chromium nitrate nonahydrate, 1.32g of terephthalic acid, 2.2mL of glacial acetic acid and 40mL of distilled water in a beaker, stir at room temperature for 30min, add 0.068mL of acrylic acid, stir for 30min, and mix The solution was poured into a hydrothermal reaction kettle, hydrothermally reacted at 200°C for 9 hours, cooled to room temperature, and washed with DMF, ethanol and distilled water in sequence to obtain acrylic acid-modified MIL-101, which was designated as AA-MIL-101.
[0038]2) Dissolve 0.3g AA-MIL-101 in 40mL deionized water, heat up to 60°C, add 0.2mL of acrylic acid to react for 20-30min, add initiator potassium persulfate 0.08g, heat up to 80°C, under nitrogen protection React for 1 h, cool to room temperature, wash with deionized water unti...
Embodiment 2
[0043] Example 2 The adsorption effect of P-AA-MIL-101 on Sc(III) and heavy metal Pb(II) under different acidity
[0044] 1. Method: Weigh 5 parts of 10mgP-AA-MIL-101 respectively, add 10mL to a concentration of 50mg·L -1 The Sc(III) solution, the pH of the Sc(III) solution is 1, 2, 3, 4, 4.5, respectively, shaken at 30°C, 180r / min in a shaking box for 24h. The result is as Figure 5a .
[0045] Depend on Figure 5a It can be seen that with the increase of pH value, the adsorption rate of P-AA-MIL-101 to Sc(III) gradually increases, and at pH=4.5, the adsorption rate of Sc(III) can reach 91%, thus realizing Recovery of rare earth element scandium.
[0046] 2. Method: Weigh 5 parts of 10mgP-AA-MIL-101 respectively, add 10mL to make the concentration 25mg·L -1 The Pb(II) solution, the pH of the Pb(II) solution is 1, 2, 3, 4, 5, shaken at 30°C, 180r / min in a shaking box for 24h. The result is as Figure 5b .
[0047] Depend on Figure 5b It can be seen that with the incr...
Embodiment 3
[0048] Example 3 The separation effect of P-AA-MIL-101 on Sc(III) in the solution of 16 kinds of mixed rare earth ions
[0049] Method: pipette 10mL respectively with a concentration of 20mg·L -1 The mixed solution of 16 kinds of rare earth elements (La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Y, Sc) were adjusted to pH 1, 2, 3, 4, 4.5, add 10mg of adsorbent P-AA-MIL-101, shake at 30°C for 24 hours, then measure the concentration of each rare earth ion in the solution. The result is as Figure 6 .
[0050] Depend on Figure 6 It can be seen that in the whole acidity range, P-AA-MIL-101 has a certain adsorption effect on 16 kinds of rare earth elements, among which the adsorption effect on Sc(III) is the best. When the pH is 3, the adsorption effect on Sc(III) The adsorption rate can reach 95.14%, by Figure 6 It can be seen that the selectivity to Sc(III) is also relatively good.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com