A preparing method of p-nitroacetophenone
A technology of p-nitroacetophenone and p-nitroethylbenzene, which is applied in the field of biomimetic catalytic oxygen oxidation of p-nitroethylbenzene to prepare p-nitroacetophenone, can solve the problem of difficult scale-up to realize industrial production and scale-up industrial production , Oxygen loss and other problems, to avoid the danger caused by high temperature, improve oxidation efficiency, and shorten the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
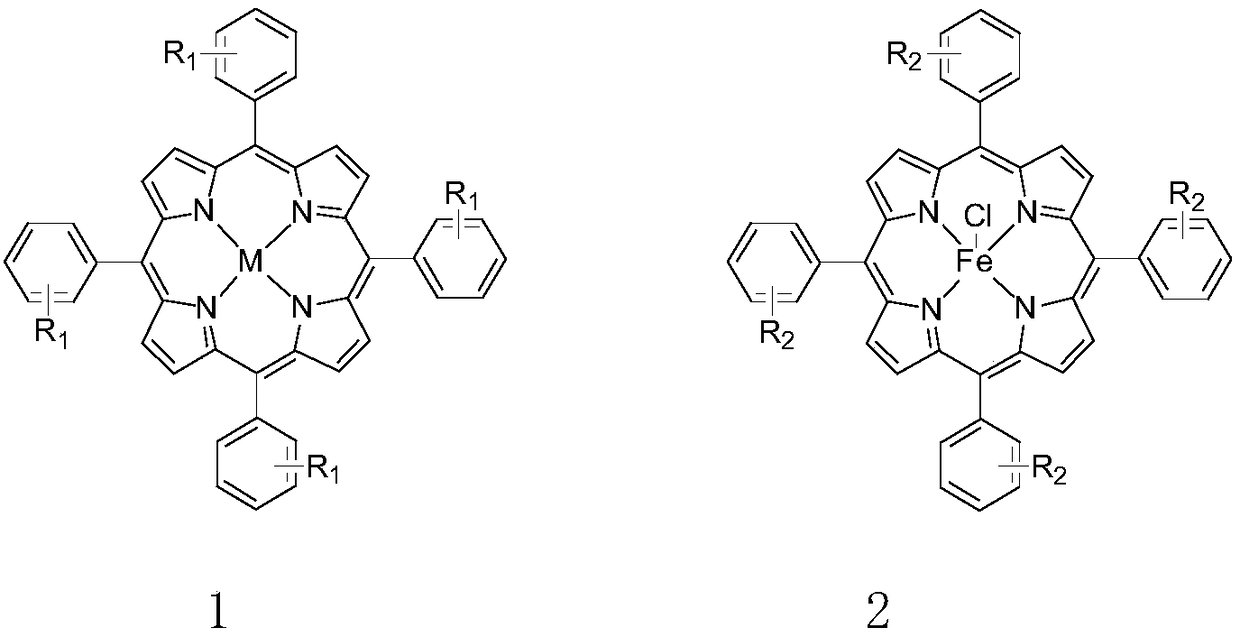
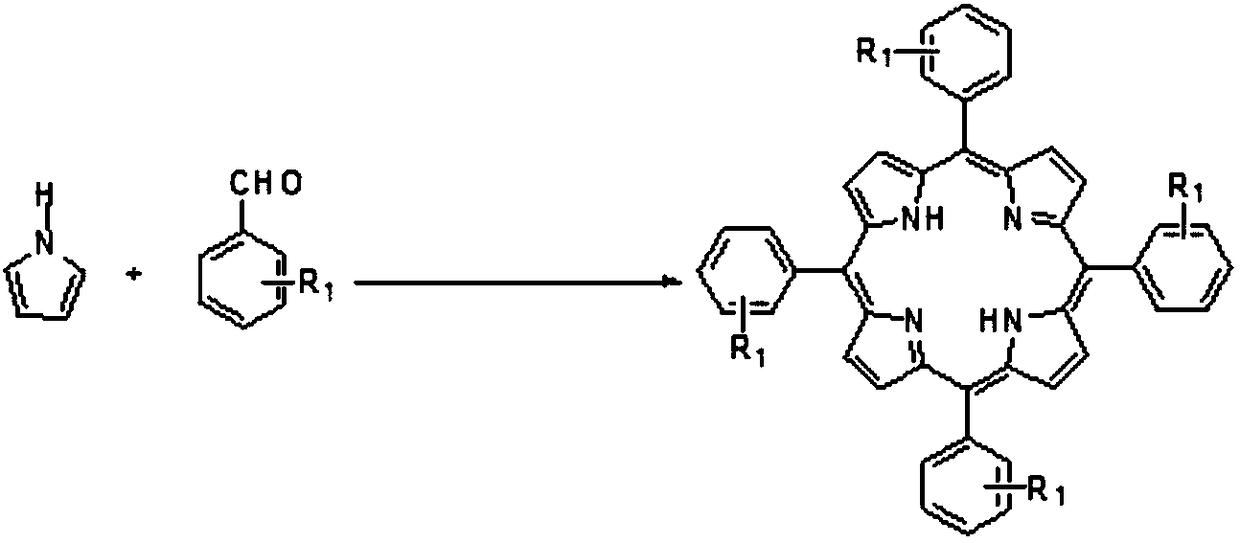
[0041] Metalloporphyrin catalyst of the present invention is specifically prepared according to the following steps:
[0042] T(p-Cl)PPFeCl:
[0043]
[0044] Take a 100mL three-necked flask, pump nitrogen three times, then add 10mmol (1.3615g) p-chlorobenzaldehyde and 10mmol (0.6709g) pyrrole, 20mL propionic acid and 20 mL acetic acid as solvent, 5 μL trifluoroacetic acid as catalyst, 2 mL nitrobenzene As an oxidant, react at 140°C for 4h, then cool to room temperature, add 100mL of methanol, then filter, wash the filter cake with methanol, and recrystallize the filter cake with methanol to obtain the ligand 450mgT(p-Cl)PP, The yield is 6.1%, then take 0.27mmol (200mg) of the above-mentioned ligand T(p-Cl)PP in a 100mL three-necked round-bottomed flask, then add 2.7mmol (438.0mg) anhydrous ferric trichloride, and replace the nitrogen three times , add the solvent DMF, react at 140°C for 12h, remove the solvent by distillation under reduced pressure with an oil pump, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com