Benzodiazepine* derivative and salts thereof and related crystal forms, preparation methods and uses thereof
A crystal and form technology, applied in the field of benzodiazepine* derivatives and their salts and related crystal form, preparation and application, can solve the problems of drug-drug interaction, long time recovery, etc., and achieve solvent residue Low, good solid state stability, the effect of ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0224] 3-(8-Chloro-1-cyclopropyl-6-(2-fluorophenyl)-4H-benzo[f][1,2,4]triazolo[4,3-a][1 ,4] diazepine -4-base) the preparation of methyl propionate (compound of formula (I))
[0225]
[0226] The first step: 7-chloro-5-(2-fluorophenyl)-1H-benzo[e][1,4]diazepine - Preparation of 2(3H)-thione (compound B)
[0227] 7-Chloro-5-(2-fluorophenyl)-1H-benzo[e][1,4]diazepine -2(3H)-one (compound A, 5.0 g, 17.3 mmol) was dissolved in tetrahydrofuran (150 mL). Phosphorus pentasulfide (5.77g, 6.55mmol) was added, the mixture was heated to 78°C and reacted for 2 hours. Filtered, washed 3 times, added water to the filtrate, extracted 3 times with ethyl acetate, dried and concentrated, purified by column chromatography to obtain the target product 7-chloro-5-(2-fluorophenyl)-1H-benzo[ e][1,4]diazepine -2(3H)-thione (compound B, 4.2 g, yield: 79%).
[0228] MS m / z(ESI):305[M+H]+
[0229] The second step: 8-chloro-1-cyclopropyl-6-(2-fluorophenyl)-4H-benzo[f][1,2,4]triazole[4,3-a]...
Embodiment 2
[0237] 3(S)-3-(8-chloro-1-cyclopropyl-6-(2-fluorophenyl)-4H-benzo[f][1,2,4]triazolo[4,3 -a][1,4]diazepine Preparation of -4-yl) methyl propionate (compound of formula (Ia))
[0238]
[0239] The first step: (S)-5-((2-fluoro-benzoyl-4-chlorophenyl) amino)-4-((tert-butoxycarbonyl) amino)-5-oxopentanoic acid methyl ester ( Preparation of compound c)
[0240] 2-amino-5-chloro-2'-fluorobenzophenone (compound a, 20g, 0.083mol, commercially available) and N-tert-butoxycarbonyl-L-glutamic acid-5-methyl ester (compound b, 23 g, 0.088 mol, commercially available) was dissolved in dichloromethane (300 mL). The mixture was cooled to 0° C., dicyclohexylcarbodiimide (DCC, 18.2 g, 0.088 mmol) was added, and stirred for 24 hours. The liquid phase mass spectrometry (LCMS) test showed that the reaction was complete. The reaction solution was poured into ice water, extracted with ethyl acetate, the organic phase was washed 3 times with water, dried and concentrated to obtain the crude p...
Embodiment 3
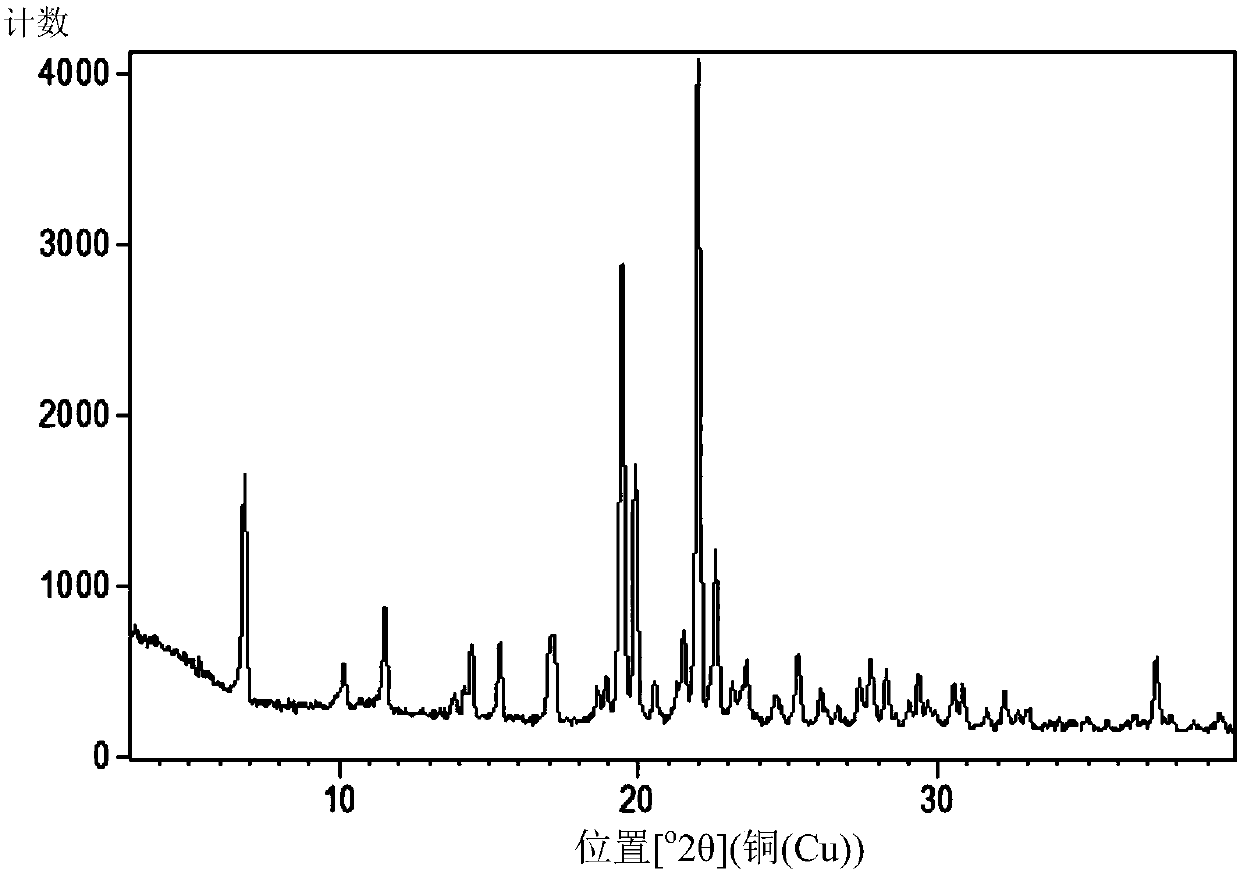
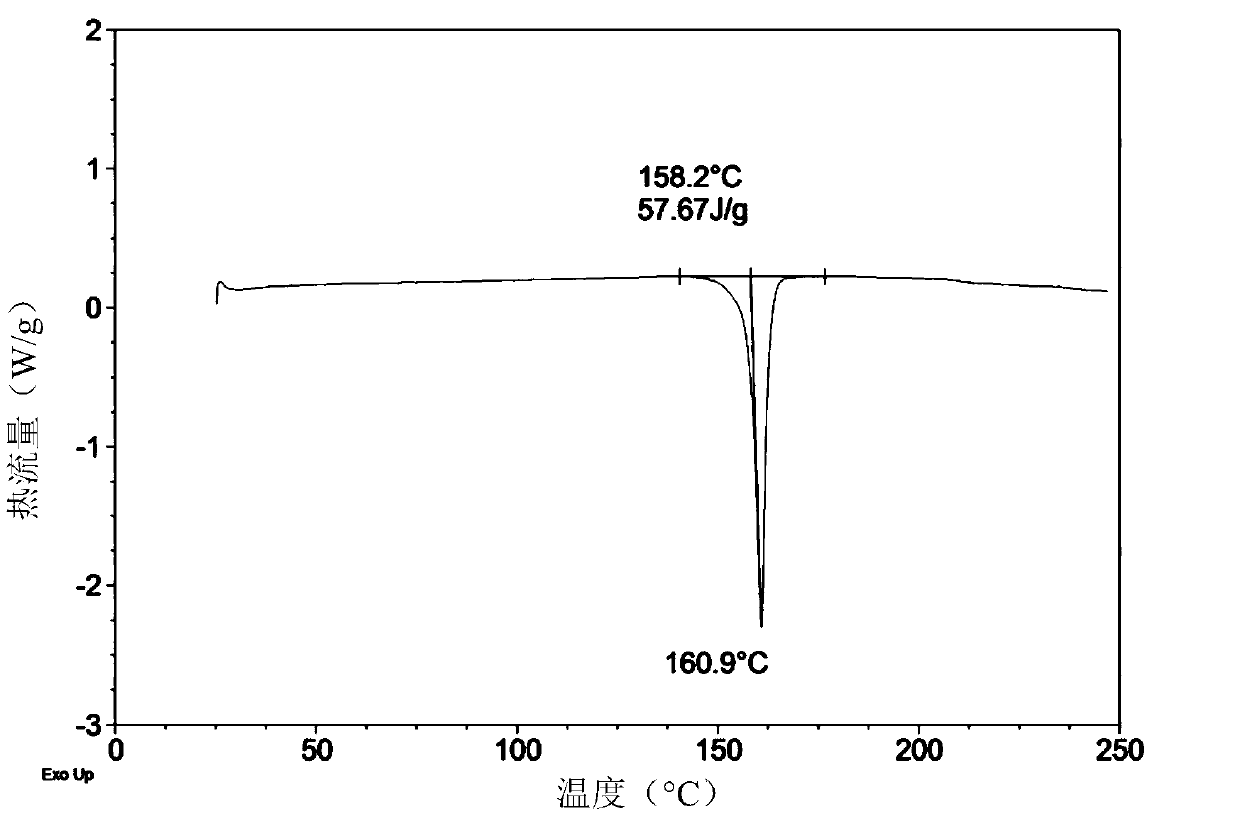
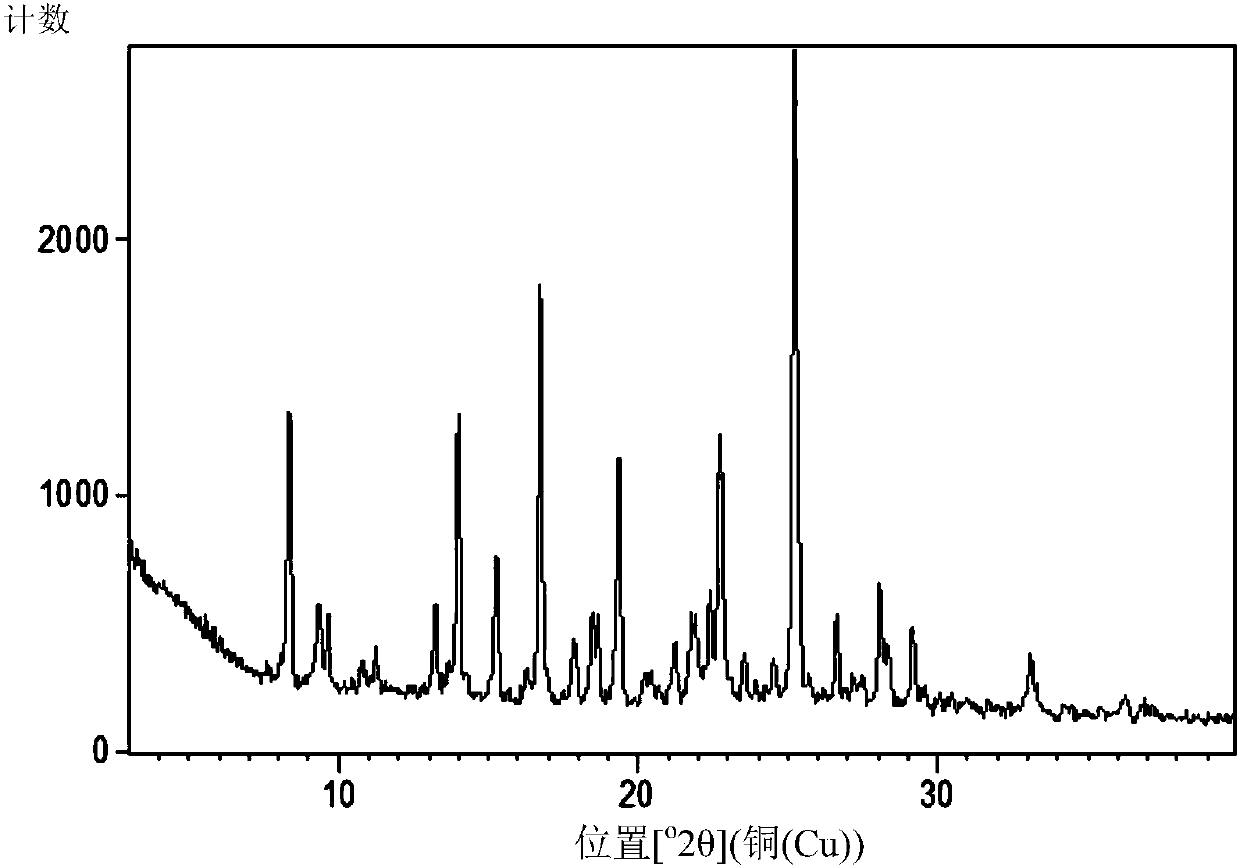
[0253] Preparation and Characterization of Crystal B of Compound of Formula (Ia)
[0254] Crystal B of the compound of formula (Ia) was prepared as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com