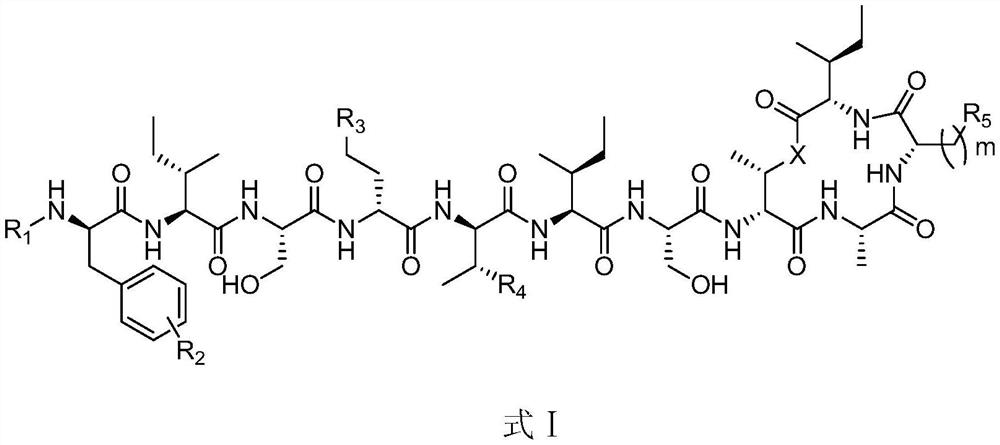
A new antibiotic for the treatment of drug-resistant Gram-positive bacteria and tuberculosis
A compound and group technology, applied in antibacterial drugs, pharmaceutical formulations, antifungal agents, etc., can solve the problems of limited clinical use and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
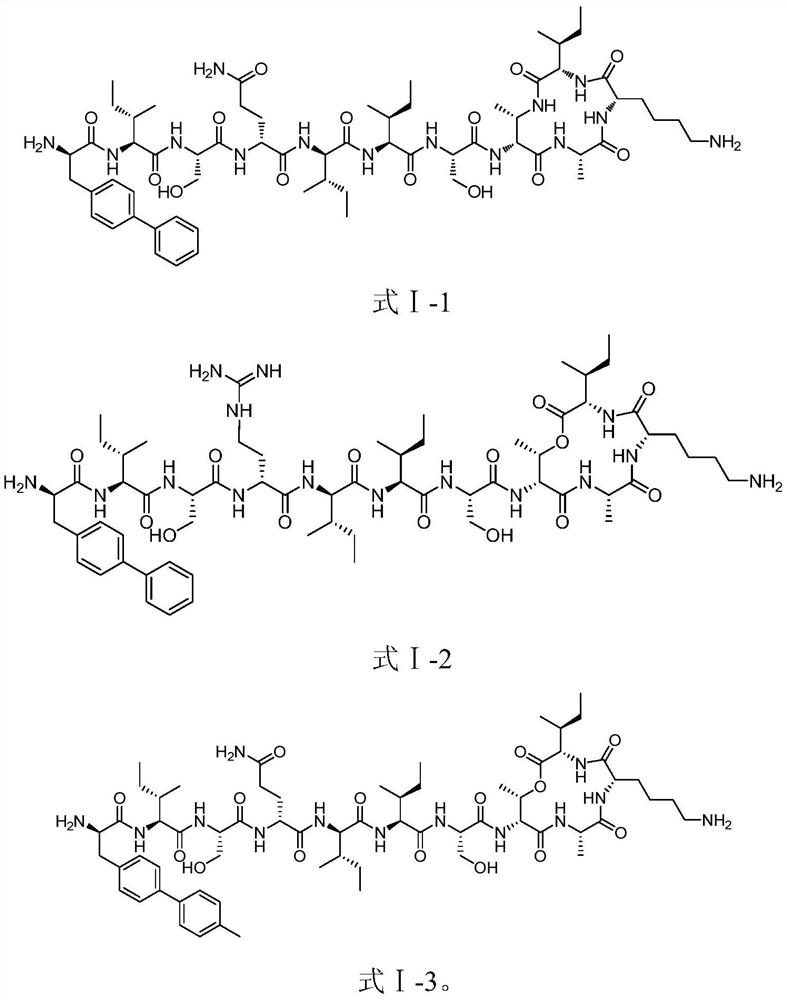
[0095] Preparation of compounds shown in embodiment 1, formula I-1, formula I-2 and formula I-3
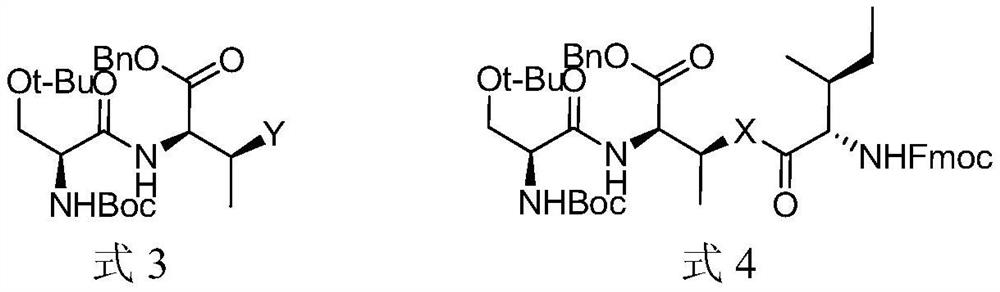
[0096] (1) Preparation of compound shown in formula 3
[0097] The reaction equation is as follows:
[0098]
[0099] Put N-Boc-O-tert-butyl-L-serine (11mmol) in a round bottom flask, add dichloromethane and N,N-dimethylformamide as a mixed solvent 50ml, HCTU (6-chlorobenzo Triazole-1,1,3,3-tetramethyluronium hexafluorophosphate) (11mmol) and DIEA (N,N-diisopropylethylamine) (11mmol) were added to the reaction solution, and then the formula The compound shown in 1-1 (10 mmol) was stirred at room temperature for 3 hours, and the reaction was quenched by adding dilute hydrochloric acid. Add 100ml of dichloromethane to dilute the reaction solution, wash with sodium bicarbonate and saturated aqueous solution of sodium chloride respectively, distill off the dichloromethane under reduced pressure, and separate the obtained product on a silica gel column (petroleum ether: ethyl acet...
Embodiment 2
[0158] The active test of the compound shown in embodiment 2, formula I-1, formula I-2 and formula I-3
[0159] Compounds were tested for MIC according to CLSI guidelines.
[0160] THY medium was selected for bacterial cultivation, and MHB medium was selected for testing. The medium used was supplemented with 0.002% Tween 80 to inhibit drug attachment. Cell concentration adjusted to 5 x 10 5 per milliliter. The concentration of the compound from high to low is 8 μg / ml, 4 μg / ml, 2 μg / ml, 1 μg / ml, 0.5 μg / ml and 0.25 μg / ml. The drug concentration is the minimum inhibitory concentration.
[0161] The test results are shown in Table 1.
[0162] MIC (μg / ml) of each compound in Table 1
[0163]
[0164]
[0165] It can be seen from the data in Table 1 that the antibacterial activity of the compound prepared by the present invention is equivalent to that of teixobactin, and the molecular structure and synthesis cost are lower.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com