A kind of vinca alkaloid drug carrier and preparation method thereof
A vinca alkaloid and carrier technology, which can be used in drug combinations, pharmaceutical formulations, antitumor drugs, etc., can solve problems such as unsatisfactory drug loading and unstable drug encapsulation efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
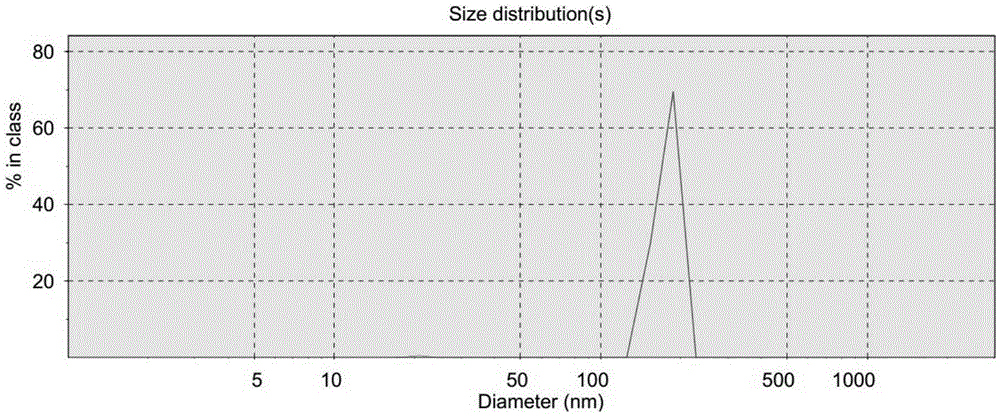
Image
Examples
example 1
[0022] Example 1 (preparation of vinca alkaloid drug carrier):
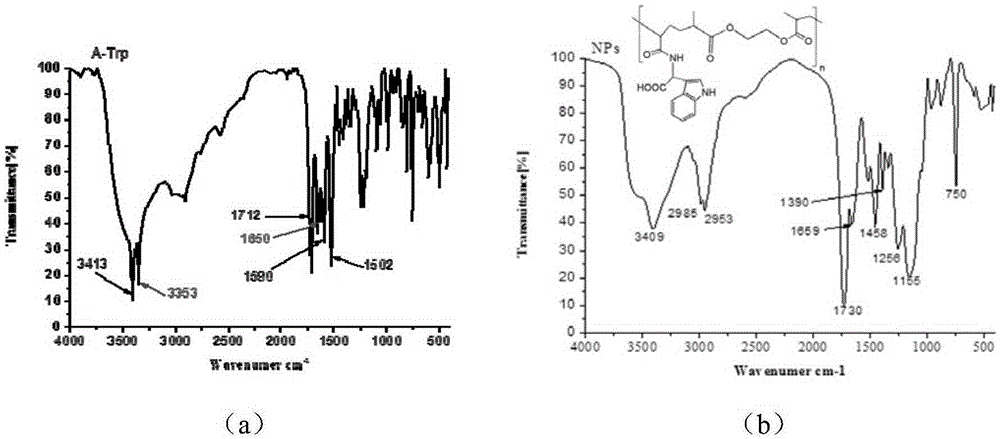
[0023] 1. Preparation of functional monomer N-acryloyl-L-tryptophan
[0024] Weigh 4.90g (0.024mol) of L-tryptophan (L-Trp) and 1.92g (0.048mol) of sodium hydroxide, dissolve in 40mL of water, and when the mixed solution becomes clear and transparent, place it on ice below 0°C in a salt bath. Under magnetic stirring, 2.00 mL (0.025 mol) of acryloyl chloride was added dropwise with a pipette. After the dropwise addition, it was stirred at room temperature for 24 h to make the reaction complete. Adjust the pH to 2 with concentrated hydrochloric acid to completely precipitate the reaction product. Suction filtration, washing with water to neutrality, and vacuum drying at 50°C for 24 hours yielded 3.25 g of white powdery solid, the functional monomer N-acryloyl-L-tryptophan (A-Trp), with a yield of 52.4%.
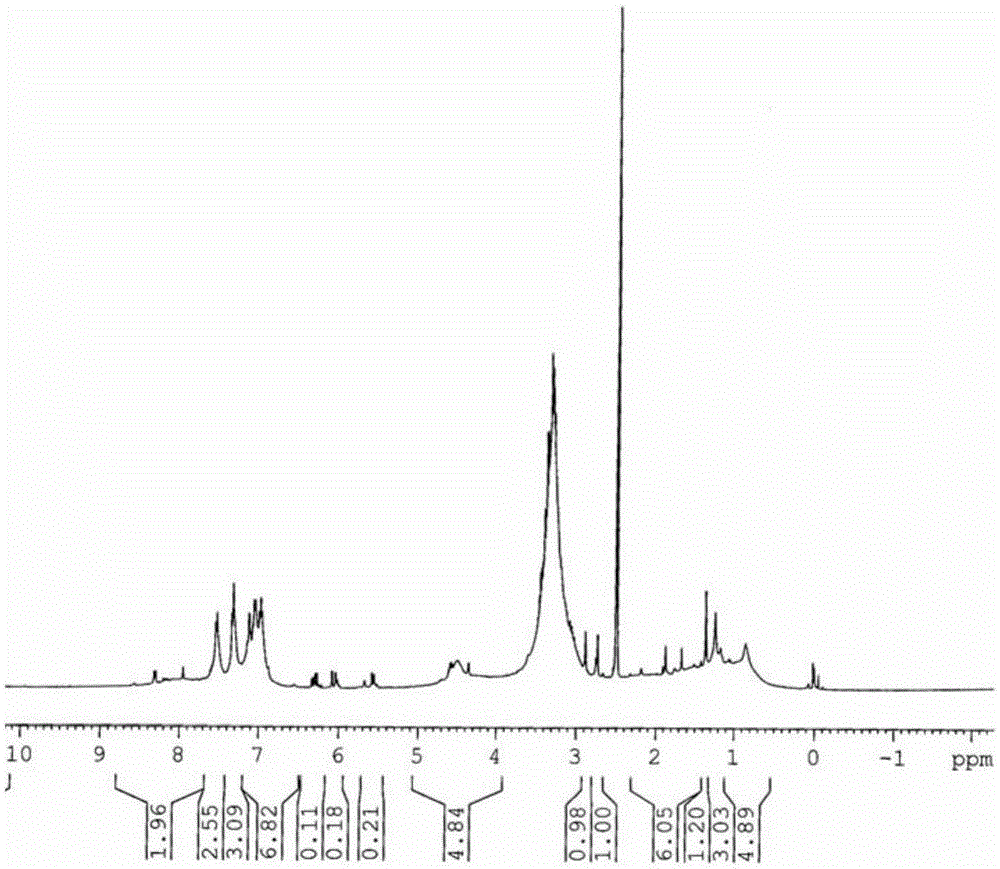
[0025] 2. Preparation of vinca alkaloid drug carrier
[0026] In a 50ml Erlenmeyer flask, add 1.8mmol func...
example 2
[0033] Example 2: (preparation of vinca alkaloid sustained-release medicine)
[0034] Weigh 5 mg of the vinca alkaloid drug carrier obtained in Preparation Example 1, place it in a 5 mL centrifuge tube, add 2 mL of vincristine solution with a concentration of 0.6 mmol / mL, place it in a shaker at room temperature and shake for 8 hours in the dark. Centrifuge at 10,000rmp for 15min with a high-speed centrifuge. Pipette the supernatant and measure the concentration of vincristine in it by ultraviolet spectroscopy, collect the precipitate, freeze-dry to obtain vincristine sulfate drug-loaded nanoparticles, and the drug loading and encapsulation efficiency are 8.55% and 65.99% respectively.
example 3
[0035] Example 3: (preparation of vinca alkaloid sustained-release medicine)
[0036] Weigh 5 mg of the vinca alkaloid drug carrier obtained in Preparation Example 1, put it into a 5 mL centrifuge tube, add 2 mL of vinblastine solution with a concentration of 0.6 mmol / mL, place it in a shaker at room temperature and shake for 8 hours in the dark. Centrifuge at 10,000rmp for 15min with a high-speed centrifuge. The supernatant was taken out and the concentration of vinblastine was determined by ultraviolet spectroscopy, and the precipitate was collected and freeze-dried to obtain vinblastine sulfate drug-loaded nanoparticles. The drug-loading capacity and encapsulation efficiency were 7.04% and 48.49%, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| cumulative release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com