Method for determining medicine or trinitride in intermediate of medicine through derivatization HPLC (high performance liquid chromatography) method
An azide compound and derivatization technology, applied in the field of drug determination, can solve the problems of inability to achieve the detection purpose, insufficient sensitivity, and difficulty in meeting requirements, and achieve the effects of increased ultraviolet absorption intensity, high sensitivity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] Preparation of biphenyl-4-acyl chloride derivatization solution: about 100 mg of biphenyl-4-acyl chloride, accurately weighed, put in a 10 mL measuring bottle, dissolved with pure acetonitrile and diluted to the mark, and shake well.
[0043] Preparation of the test solution: Take an appropriate amount of cefamandole sodium, candesartan or zidovudine, add 100 μL of derivatization reagent, dilute to the mark with acetonitrile, shake well, and react at 25°C for 30 minutes to obtain.
[0044] Preparation of reference substance solution: take about 16 mg of sodium azide reference substance, accurately weigh it, put it in a 10mL volumetric flask, dissolve it in 2mL of water, and dilute to the mark with acetonitrile; after shaking well, accurately draw 50μL into the 10mL volumetric flask , and dilute to the mark with acetonitrile. (5 μg / mL, calculated as Azide).
[0045] Chromatographic conditions: chromatographic column is C18 (5μm, 4.6*250mm), with octadecylsilane bonded s...
Embodiment 1
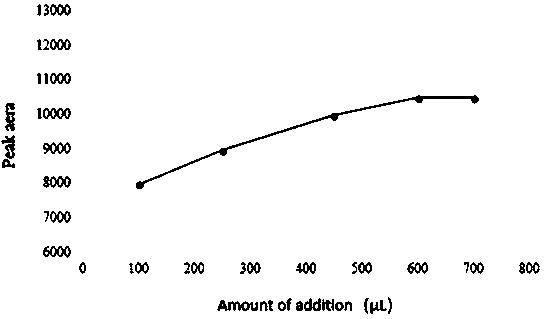
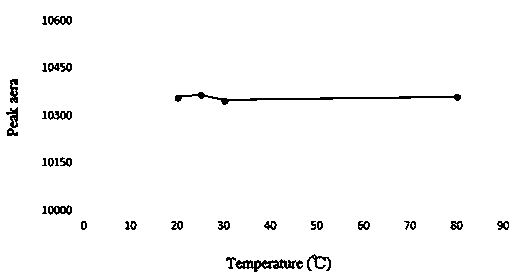
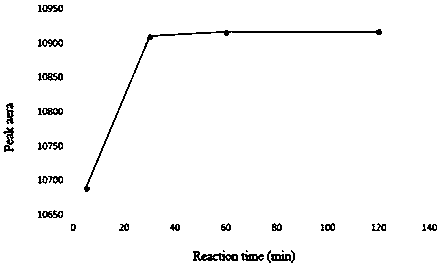
[0047] In order to ensure the complete derivatization reaction, as represented by candesartan, it is necessary to investigate the amount of derivatization reagent in the system, the reaction time and the reaction temperature. For specific results, see Figure 1-Figure 3 .
[0048] Depend on figure 1 It can be seen that the peak area of the derivatized product increases with the increase of the amount of derivatization reagent, until the amount of biphenyl-4-formyl chloride is 600 μL, the peak area reaches the maximum and remains unchanged. Depend on figure 2 It can be seen that increasing the reaction temperature has little effect on the peak area of the derivatized product, indicating that the derivatization reaction can proceed rapidly at room temperature. Depend on image 3 It can be seen that the reaction time of 30min can complete the derivatization reaction.
Embodiment 2
[0050] Methodology Validation and Application
[0051] Exclusive experiment:
[0052] The derivatization reactions of cefamandole sodium raw material, zidovudine raw material and candesartan raw material were respectively investigated, and the investigation showed that the blank solution did not interfere with the target derivative, and the azide derivative peak and the adjacent peak Resolution ≥ 1.5, indicating that the specificity of the method is good, specific results refer to Figure 4 ~ Figure 6 shown.
[0053] Linearity and range:
[0054] Precisely pipette an appropriate amount of azide stock solution, place it in a 10mL volumetric flask, dilute to the mark with acetonitrile, prepare a series of concentration solutions, perform derivatization according to the above method, filter through a 0.22μm syringe filter, and take 20μL Inject into a liquid chromatograph, and measure the peak area of the derivatized product. Taking the azide concentration (ng / ml) as the abs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com