Preparation method and application of a colorectal localized drug release pharmaceutical composition and its preparation
A colorectal and composition technology, which is applied in the field of medicine, can solve the problems that it is impossible to have a significant improvement, the change of the dissolution of the sample after acid and alkali is not investigated, and the characteristics of colorectal localized release are not considered.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
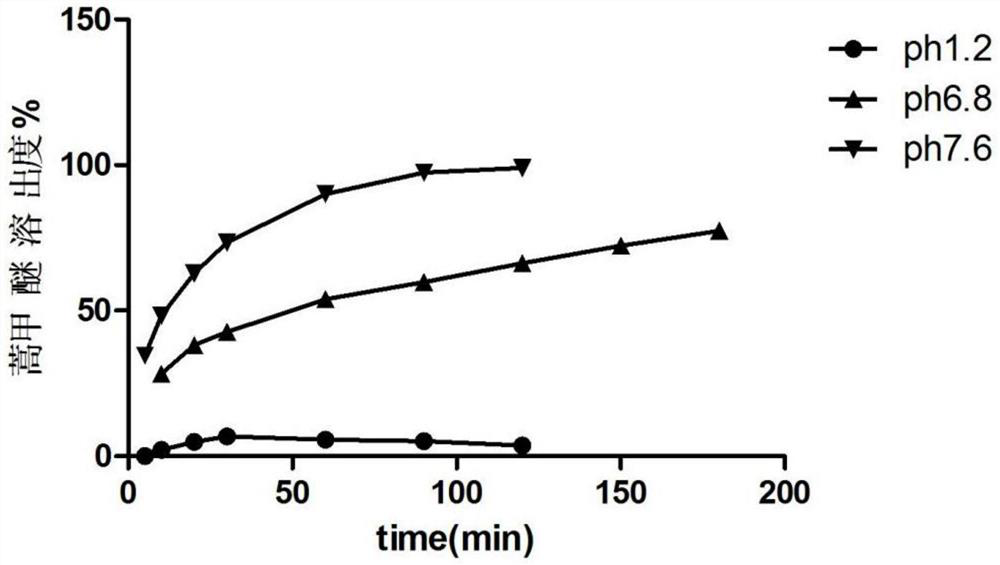
[0065] Example 1, Preparation of artemether colorectal localized drug release solid dispersion tablet
[0066] Prescription composition:
[0067]
[0068] Completely dissolve hypromellose acetate succinate (HPMCAS-HF) in 300ml of 80% ethanol, completely dissolve artemether with an appropriate amount of 95% ethanol, and add the ethanol to HPMCAS-HF under the condition of mechanical stirring 500rpm / min solution, continue to stir for 30min. The mixed solution is spray-dried with a spray dryer, and the operating parameters are: set inlet temperature: 50°C, maximum pumping speed percentage: 100%, nitrogen flow rate: 30mm, feed rate: 15% (5mL / min), condensation temperature : 0 ℃, spray dry to obtain artemether colorectal localized drug release solid dispersion. Take the dispersion and add hypromellose aqueous solution to prepare wet granules, dry; mix lactose, microcrystalline cellulose, and sodium carboxymethyl starch evenly, add hypromellose aqueous solution to prepare wet gr...
Embodiment 2
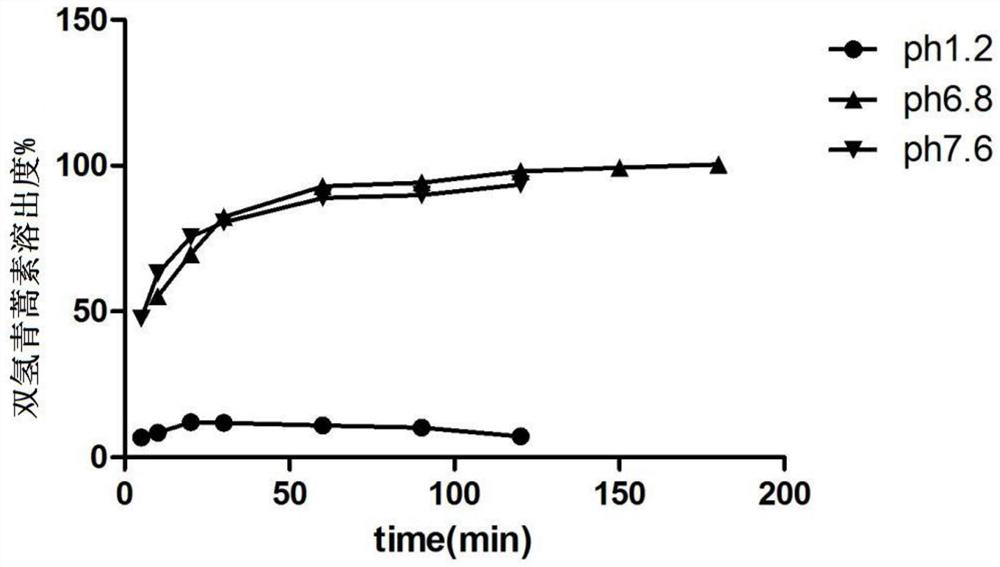
[0069] Example 2, Preparation of Dihydroartemisinin Colorectal Targeted Release Solid Dispersion Tablets
[0070] Prescription composition:
[0071]
[0072] Completely dissolve hypromellose acetate succinate (HPMCAS-HF) in 350ml of 80% ethanol, completely dissolve dihydroartemisinin with an appropriate amount of 95% ethanol, and add it to HPMCAS-HF under the condition of mechanical stirring 500rpm / min In the ethanol solution, continue to stir for 30min. The mixed solution is spray-dried with a spray dryer, and the operating parameters are: set inlet temperature: 50°C, maximum pumping speed percentage: 100%, nitrogen flow rate: 30mm, feed rate: 15% (5mL / min), condensation temperature : 0°C, spraying and drying to obtain the solid dispersion of dihydroartemisinin for colorectal targeted release. Take the dispersion and add carboxymethylcellulose sodium aqueous solution to prepare wet granules, and dry; mix mannitol, microcrystalline cellulose, and carboxymethyl starch sodi...
Embodiment 3
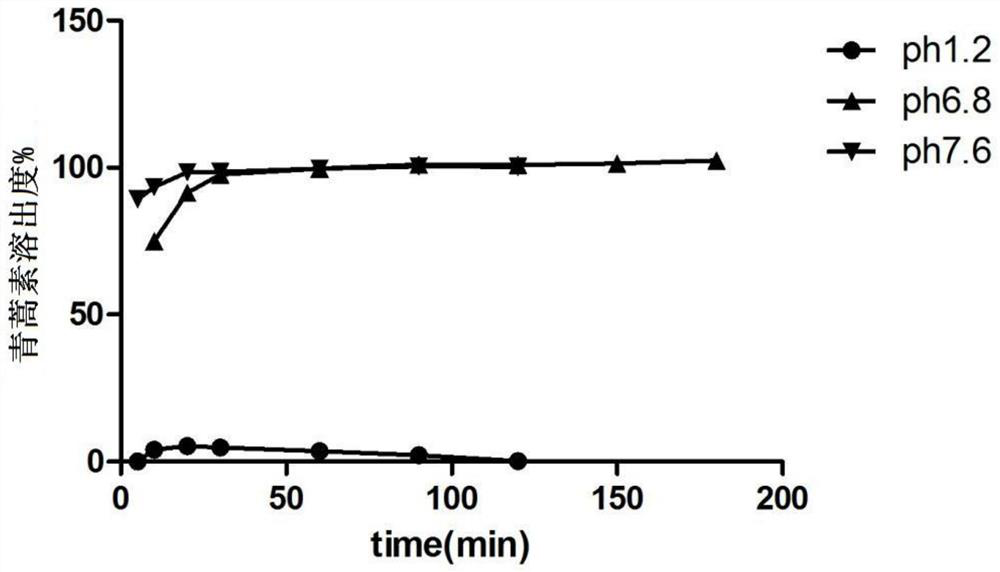
[0073] Example 3, Preparation of Artemisinin Colorectal Targeted Release Solid Dispersion Tablets
[0074] Prescription composition:
[0075]
[0076]
[0077] Completely dissolve methacrylic acid and methyl methacrylate (1:2) in 600ml of 80% ethanol, completely dissolve artemisinin with an appropriate amount of 95% ethanol, and add methacrylic acid and methyl methacrylate under the condition of mechanical stirring at 500rpm / min. In the ethanol solution of methyl acrylate (1:2), continue to stir for 30min. The mixture was spray-dried with a spray dryer. The operating parameters are: set the inlet temperature: 50°C, the maximum pumping speed percentage: 100%, the nitrogen flow rate: 30mm, the feed rate: 15% (5mL / min), the condensation temperature: 0°C, and spray dry to obtain artemisinin Solid dispersion for colorectal localized drug release. Take the dispersion and add carboxymethylcellulose sodium aqueous solution to prepare wet granules, dry; mix sucrose, starch, de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| release amount | aaaaa | aaaaa |
| release amount | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com