Fluorescent functional monomer, molecularly imprinted polymer, and preparation method and application thereof
A technology of functional monomers and molecular imprinting, applied in fluorescence/phosphorescence, chemical instruments and methods, and material analysis through optical means, can solve problems such as human poisoning, strong irritation, and body dysfunction, and achieve improved sensitivity and Effects of selectivity, fluorescence intensity enhancement, and production cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 1. Preparation of fluorescent functional monomers
[0054] (1) Preparation of fluorescent functional monomers
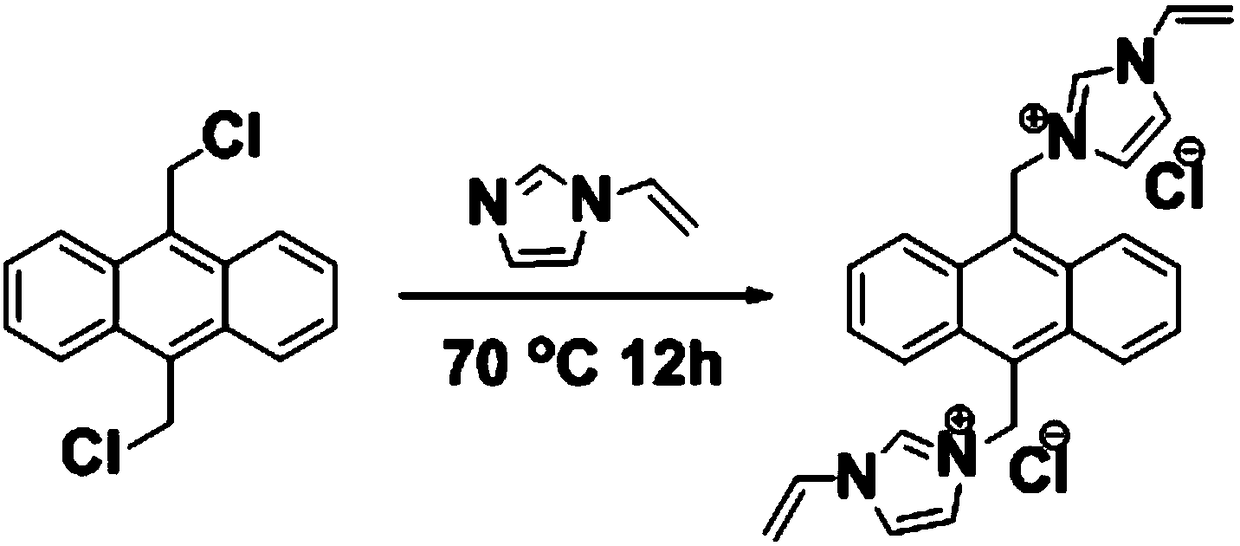
[0055] figure 1 For the reaction flow chart, weigh 9,10-bischloromethylanthracene (1.375g) and 1-vinylimidazole (1.128g) and dissolve them in 30mL of anhydrous acetonitrile, heat up to 70°C, and keep at this temperature to condense and reflux 12h, after cooling, a light yellow solid was obtained. Then filter the obtained solid, and after repeated filtration and washing with anhydrous ether for 5 times, put the light yellow solid in a vacuum drying oven and dry it to constant weight, which is the fluorescent functional monomer—9,10-bis(1-vinyl- 3-imidazolium chloride) methyl anthracene.
[0056] The mass-to-charge ratio of the synthesized fluorescent functional monomer is 391.1854 (M-2Cl - ), showing that the molecular weight of the functional monomer is 462.1378.
[0057] Its H NMR and C NMR data are as follows:
[0058] 1 H NMR (400MHz, CD 3 OD): δ=5....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com