Preparation method for tripolymerized indenyl BODIPY-fullerene starlike compound
A technology of trisindenyl and fullerene, which is applied in the field of organic synthesis, can solve the problems of complex reaction, many synthesis steps, and low yield, and achieve the effects of high-efficiency energy/electron transfer and strong light absorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
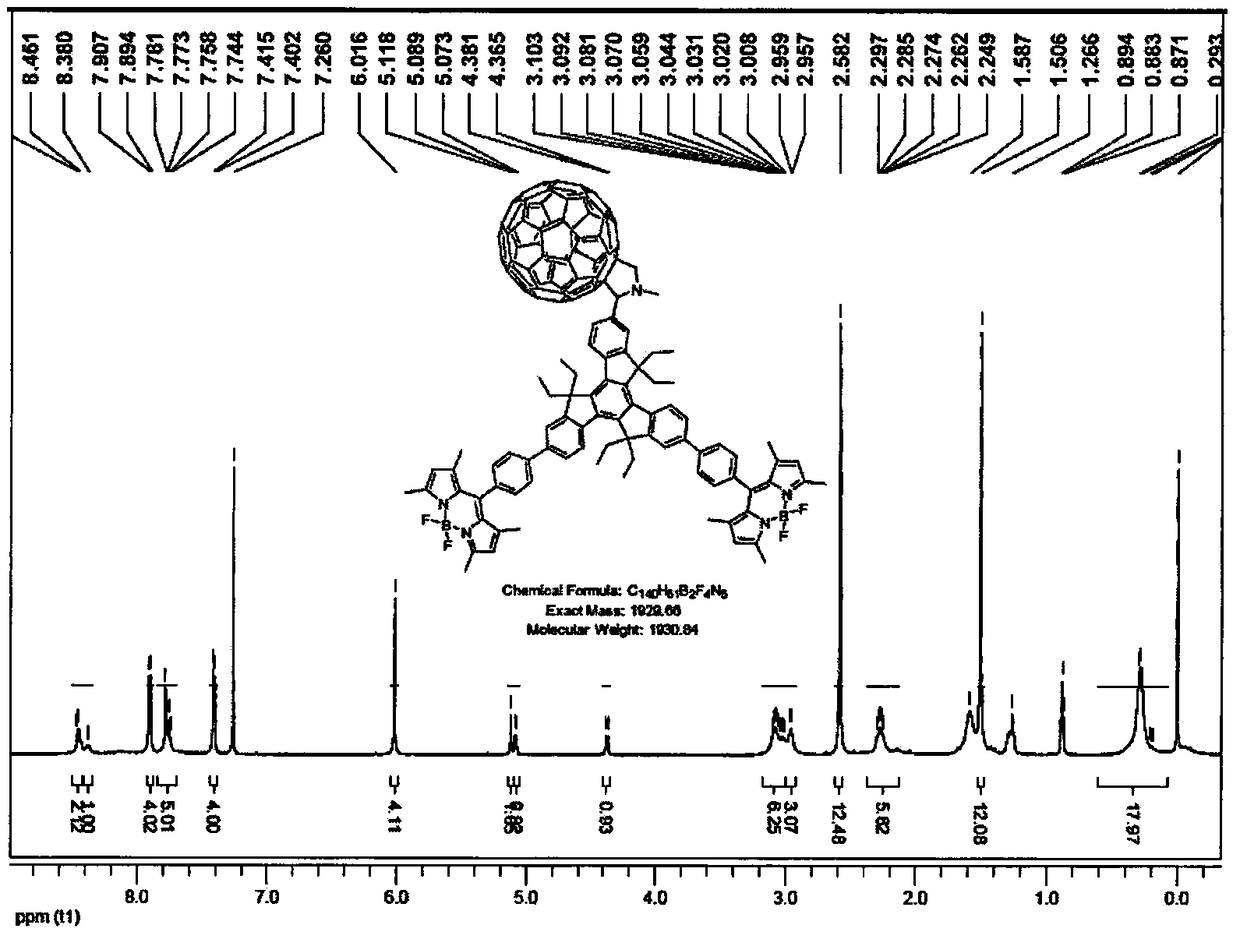
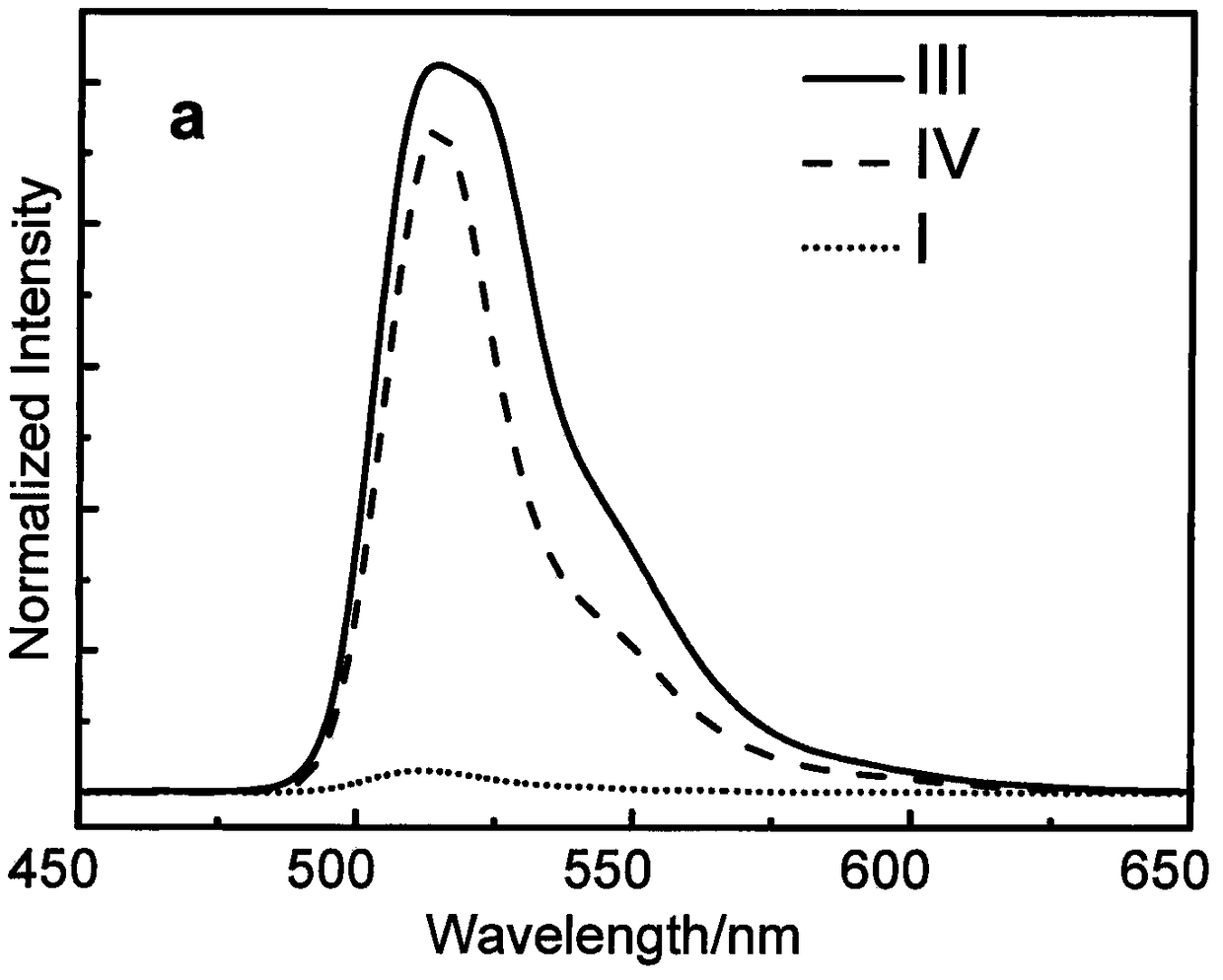
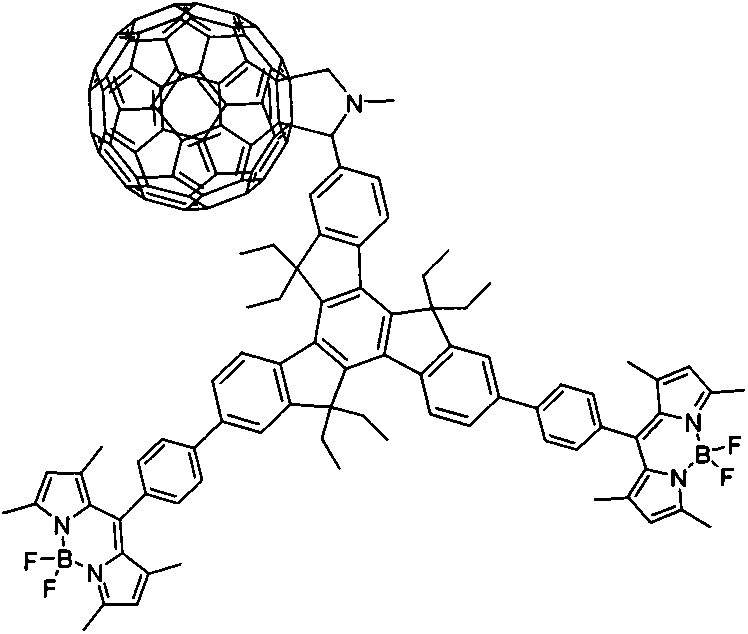
[0028] Under argon protection conditions, meso-phenylboronic acid ester BODIPY derivative (III) (1.20mmol, 540.2mg), 7,12-dibromotriindaldehyde derivative (II) (0.60mmol, 417.9mg) and anhydrous sodium carbonate (21.60mmol, 2.28g) were added to the reaction flask, tetrahydrofuran (70mL), methanol and water (v / v, 3.0mL / 3.0mL) were added, and tetrakis(triphenylphosphine) palladium ( 0.12mmol, 138.6mg), heated to 70°C and refluxed for 24h. After the reaction, cooled to room temperature, added saturated ammonium chloride solution, extracted the crude product with dichloromethane, combined the organic layers and dried over anhydrous sodium sulfate. Recover the solvent by distillation under reduced pressure, and use dichloromethane-petroleum ether (volume ratio 1:2) as the eluent to obtain 84.4 mg of tripolyindenyl BODIPY intermediate (IV) through silica gel chromatography column separation, yield: 12%. UV-vis (CH 2 Cl 2 ), λ max / nm[λ×10 -5 (L·mol -1 cm -1 )]: 330 (0.13579), ...
Embodiment 2
[0031] Under argon protection conditions, meso-phenylboronic acid ester BODIPY derivative (III) (1.20mmol, 540.2mg), 7,12-dibromotriindaldehyde derivative (II) (0.60mmol, 417.9mg) and anhydrous sodium carbonate (21.60mmol, 2.28g) were added to the reaction flask, tetrahydrofuran (70mL), methanol and water (v / v, 2.0mL / 2.0mL) were added, and tetrakis(triphenylphosphine) palladium ( 0.06mmol, 69.3mg), heated to 70°C and refluxed for 24h. After the reaction, cooled to room temperature, added saturated ammonium chloride solution, extracted the crude product with dichloromethane, combined the organic layers and dried over anhydrous sodium sulfate. Recover the solvent by distillation under reduced pressure, and use dichloromethane-petroleum ether (volume ratio 1:2) as the eluent to separate through silica gel chromatography to obtain the tripolyindenyl BODIPY intermediate (IV), with a yield of 10 %.
[0032] Under the protection of argon, tripolyindenyl BODIPY intermediate (IV) (0.0...
Embodiment 3
[0034] Under argon protection conditions, meso-phenylboronic ester BODIPY derivative (III) (1.38mmol, 621.0mg), 7,12-dibromotriindaldehyde derivative (II) (0.60mmol, 417.9mg) and anhydrous potassium carbonate (21.60mmol, 2.98g) were added to the reaction flask, tetrahydrofuran (60mL), methanol and water (v / v, 2.5mL / 2.5mL) were added, and tetrakis(triphenylphosphine) palladium ( 0.08mmol, 92.4mg), heated to 70°C and refluxed for 24h. After the reaction, cooled to room temperature, added saturated ammonium chloride solution, extracted the crude product with dichloromethane, combined the organic layers and dried over anhydrous sodium sulfate. Recover the solvent by distillation under reduced pressure, and use dichloromethane-petroleum ether (volume ratio 1:2) as the eluent to separate through silica gel chromatography to obtain the tripolyindenyl BODIPY intermediate (IV), with a yield of 15 %.
[0035] Under the protection of argon, tripolyindenyl BODIPY intermediate (IV) (0.020...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com