Polyethylene glycol-b-polytyrosine-lipoic acid copolymer, polypeptide micelle and preparation method and application thereof
A polyethylene glycol and polytyrosine technology, applied in the field of polytyrosine materials, can solve the problems of poor tumor targeting effect, slow release, easy early release of drugs, etc., and achieve good biocompatibility and high efficiency. Load and prepare simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1 Synthesis of PEG-PTyr-LA Polypeptide Copolymer
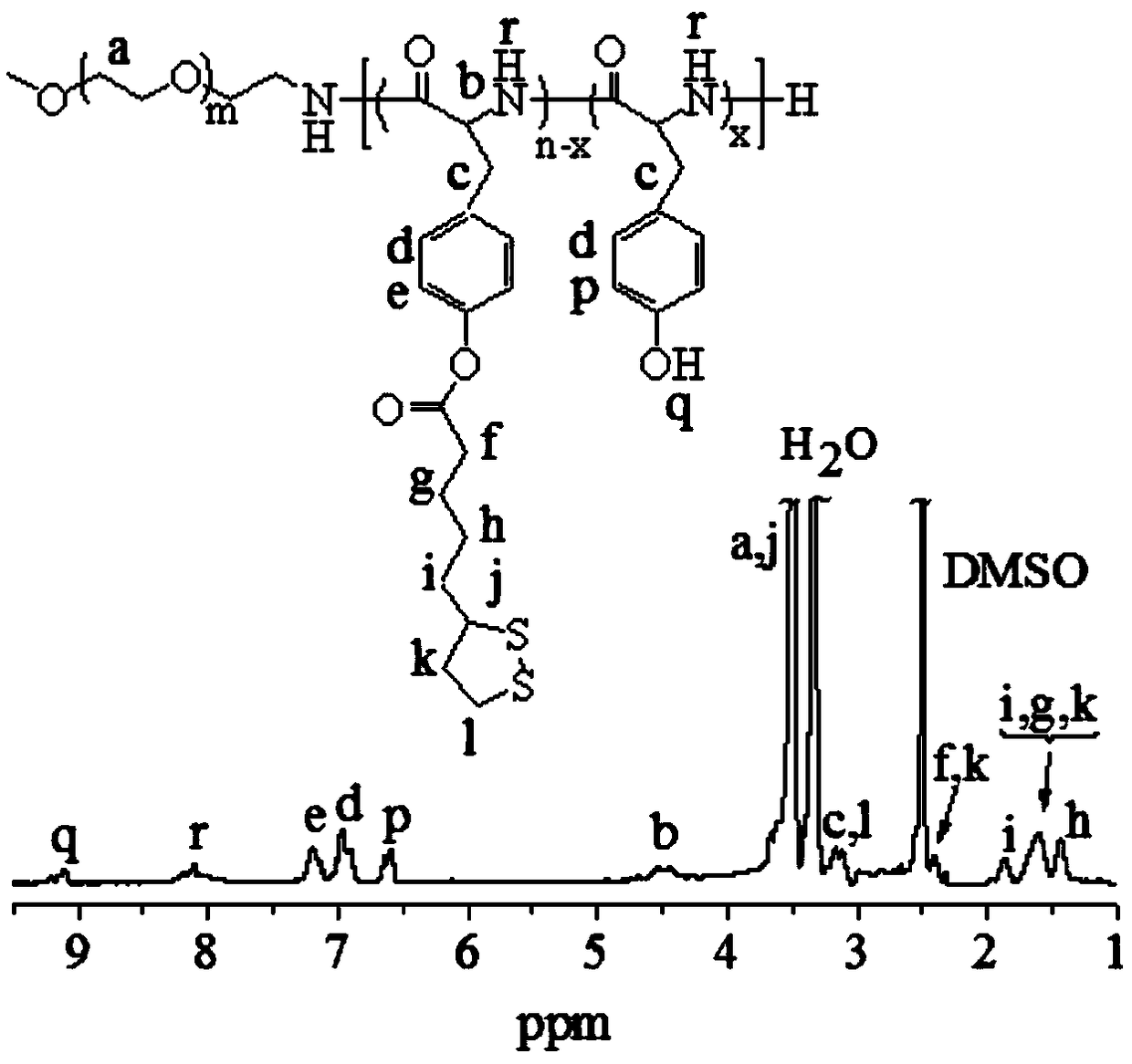
[0056] First, PEG- b -PTyr is based on PEG-NH 2 It is obtained by ring-opening polymerization of Tyr-NCA monomer as a macroinitiator. The details are as follows: Dissolve Tyr-NCA (231.0 mg, 1115.7 μmol) in 2 mL DMF, and slowly add it dropwise under nitrogen until PEG-NH is dissolved 2 (5 KDa , 400.0 mg, 80.0 μmol) in DMF (4 mL) and reacted in an oil bath at 40 °C. After 72 hours, precipitate with excess glacial ether, dissolve with chloroform after centrifugation, and repeat the precipitation twice. The precipitate was collected and dried in a vacuum oven for 24 h to obtain a white powdery polymer PEG- b -PTyr( M n : 7.0 kg / mol).
[0057] PEG- b -PTyr-LA is catalyzed by DMAP through PEG- b The phenolic hydroxyl group on the -PTyr side chain is obtained by esterification. The specific operation is as follows: lipoic acid (LA, 108.5 mg, 526.7 μmol) was dissolved in 3 mL DCM under a nitrogen atmosphere...
Embodiment 2
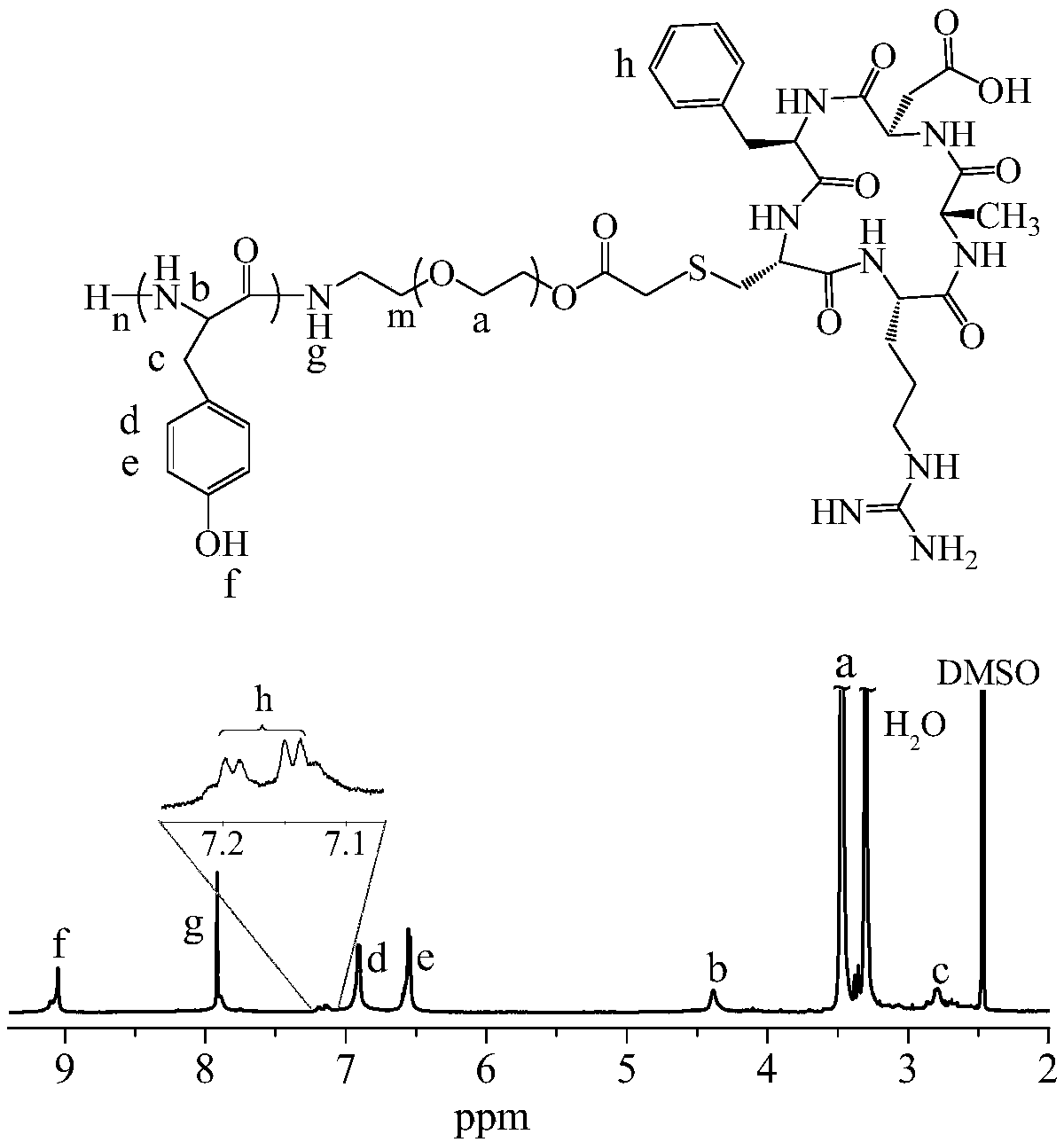
[0061] Example 2 Synthesis of cRGD-PEG-PTyr
[0062] Under nitrogen atmosphere, allyl-PEG- b -PTyr (200 mg, 28.6 μmol, see Example 1 for the preparation method, PEG-NH 2 (400.0 mg, 80.0 μmol) replaced by allyl-PEG-NH 2 ( M n =5.0 K, 400.0 mg, 80.0 μmol) in DMF solution were added thiol-modified cRGD-SH (33.9 mg, 57.1 μmol) and photosensitizer 2959 (4.0 mg, 17.8 μmol), and the mixed solution was placed in an ice bath React in a UV curing box for 15 min. The reaction solution was dialyzed with a large amount of DMF (Specture / Pore, MWCO 3500) to remove excess cRGD and 2959. Finally, the polymer was precipitated in excess anhydrous ether, and the precipitate was dried in a vacuum oven for 12 hours, yield: 70.5%.
[0063] The above preparation scheme can be expressed as follows:
[0064]
[0065] cRGD-PEG-PTyr NMR characterization see appendix figure 2 , 1 H NMR (400 MH Z , DMSO- d 6 ) 9.07 (1 H, -C 6 h 4 -OH), 7.95 (1 H, -HNCH(CH 2 )CO-), 6.91, 6.54 (4H, -C 6 h...
Embodiment 3
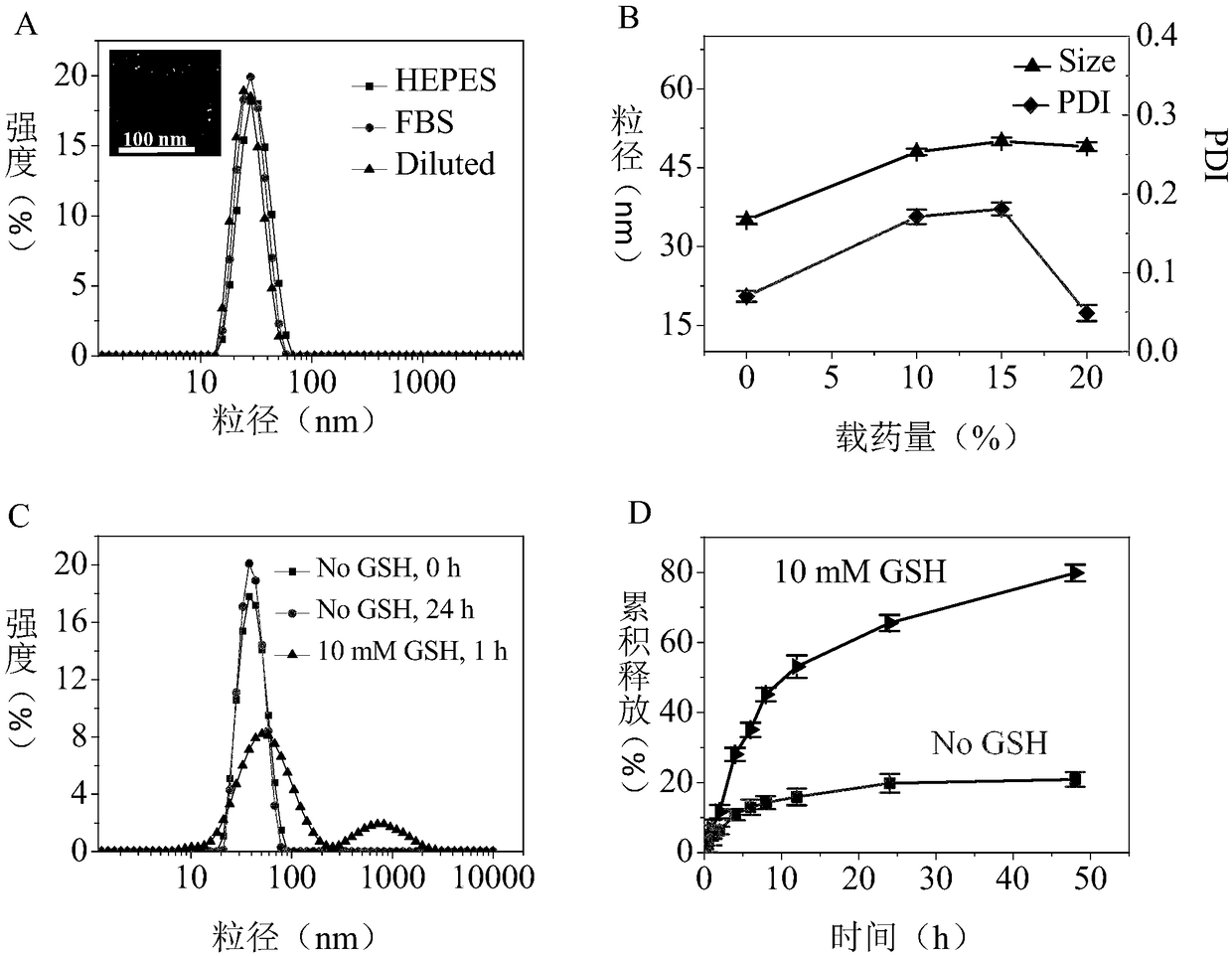
[0066] Example 3 Preparation of cRGD-rPTM-Dox polymer micelles
[0067] cRGD-modified polytyrosine micelles were prepared by dialysis, and the molar ratio of 2:8 cRGD-PEG-PTyr / PEG- b - PTyr-LA and doxorubicin Dox with a designed drug loading of 20% were dissolved in DMF; then 100 μL (10 mg / mL) of the polymer / drug solution was added dropwise to 1 mL of HEPES buffer solution at pH 7.4 , stirring at a speed of 400 rpm while adding dropwise. After stirring, the micelles / drug solution was transferred to a dialysis bag with a molecular weight cut-off of 3.5 K and dialyzed with HEPES buffer solution of pH 7.4 for 8 h, and the dialysis medium was changed every 2 h; 25 μL (1 mg / mL) of DTT solution was added to the solution, and placed in a 37︒C 100 rpm shaker for 12 h to obtain the cross-linked drug-loaded micelles cRGD-rPTM-Dox, that is, nano-drugs; the same method, without adding doxorubicin Dox with a designed drug loading of 20%, to obtain cross-linked empty micelles, denoted as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Grafting rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com