Catalyst for synthesis of hydrogen peroxide by catalyzing oxygen reduction under visible light and preparation method thereof
A technology of hydrogen peroxide and catalyst, which is applied in the field of catalyst and its preparation for catalytic oxygen reduction to synthesize hydrogen peroxide, which can solve problems such as explosion and achieve the effects of wide sources, rich content and good reaction stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
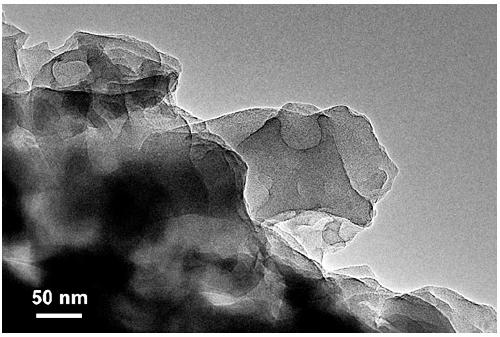
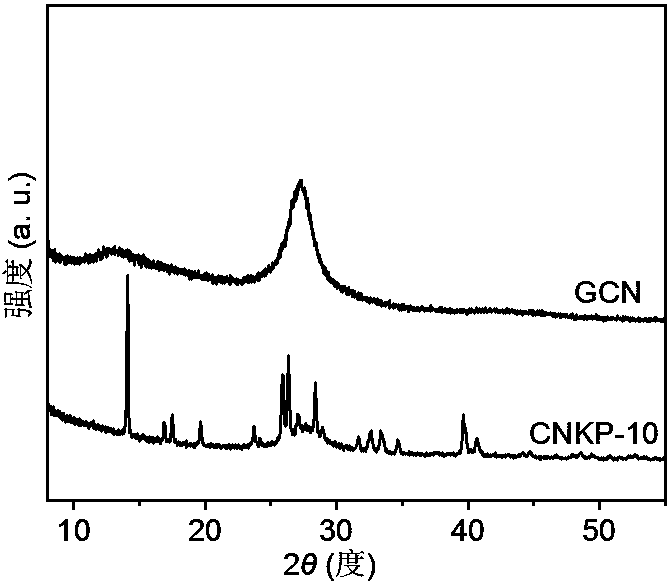
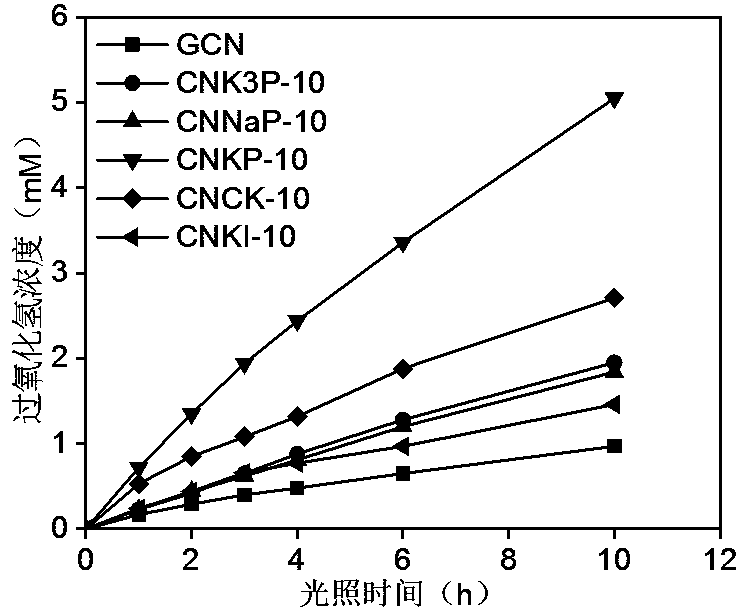
[0027] Weigh 10.0 g urea and 10 mmol dipotassium hydrogen phosphate (K 2 HPO 4 ·3H 2 O), grind well in an alumina mortar, make it well mixed, and transfer to a 50mL alumina crucible. Place the crucible containing the precursor in a muffle furnace, raise the temperature to 450°C at a rate of 3°C per minute, keep it in still air for 2h, and cool down naturally to obtain a graphite-phase carbon nitride material doped with phosphorus and potassium. Sample It is light yellow. Grind the obtained block sample evenly to make it powdery, disperse it in deionized water, the ratio of sample to water is 1 g: 1 L, peel it off under ultrasonic for 3.0 h, vacuum filter it with a 0.45 μm filter membrane, and use it to remove Repeated washing with deionized water and suction filtration several times. The sample obtained by suction filtration was dried in a vacuum oven at 60° C. for 12 hours to obtain the CNKP-10 catalyst. The TEM photomicrograph of the catalyst is shown in figure 1 , the...
Embodiment 2
[0029] Dipotassium hydrogen phosphate (K 2 HPO 4 ·3H 2 O) replaced by potassium phosphate (K 3 PO 4 ), other preparation conditions are the same as in Example 1. The resulting catalyst is designated CNK3P-10.
Embodiment 3
[0031] Dipotassium hydrogen phosphate (K 2 HPO 4 ·3H 2 O) replaced by disodium hydrogen phosphate (Na 2 HPO 4 ), other preparation conditions are the same as in Example 1. The resulting catalyst was designated as CNNaP-10.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com