Method for preparing lithium hexafluorophosphate from organic solvent
A technology of lithium hexafluorophosphate and organic solvents, which is applied in lithium hexafluorophosphate, chemical instruments and methods, phosphorus compounds, etc., can solve the problems of increasing the purification cost of lithium hexafluorophosphate, the high technical difficulty of lithium hexafluorophosphate, and the high instability of lithium hexafluorophosphate, so as to avoid thermal decomposition and respond quickly and thorough, easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
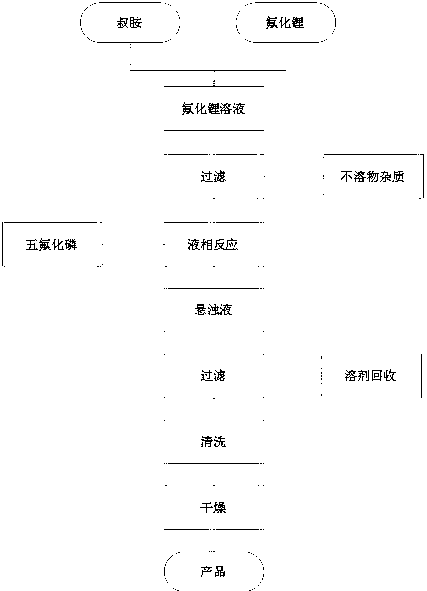
[0031] A method for preparing lithium hexafluorophosphate with an organic solvent, the steps are as follows:
[0032] (1) Grinding lithium fluoride to 400 mesh, stirring and dissolving 2.6 kg of crushed lithium fluoride in 100 liters of anhydrous triethylamine solvent;
[0033] (2) Filtrating the lithium fluoride solution to remove insoluble matter to obtain a filtrate;
[0034] (3) Pass 126 kg of phosphorus pentafluoride into the filtrate, and continue to heat and stir at 60°C for 1 hour to obtain a suspension containing the precipitated product;
[0035] (4) The suspension was filtered, and the obtained filter cake was washed with pentane, and vacuum-dried at 30° C. for 2 hours to obtain a lithium hexafluorophosphate product.
Embodiment 2
[0037] A method for preparing lithium hexafluorophosphate with an organic solvent, the steps are as follows:
[0038] (1) Grinding lithium fluoride to 400 mesh, stirring and dissolving 2.6 kg of crushed lithium fluoride in 100 liters of anhydrous tripropylamine solvent;
[0039] (2) Filtrating the lithium fluoride solution to remove insoluble matter to obtain a filtrate;
[0040] (3) Pass 189 kg of phosphorus pentafluoride into the filtrate, and continue to heat and stir at 60°C for 1 hour to obtain a suspension containing the precipitated product;
[0041] (4) The suspension was filtered, and the obtained filter cake was washed with pentane, and vacuum-dried at 10° C. for 5 hours to obtain a lithium hexafluorophosphate product.
Embodiment 3
[0043] A method for preparing lithium hexafluorophosphate with an organic solvent, the steps are as follows:
[0044] (1) Grinding lithium fluoride to 400 mesh, stirring and dissolving 2.6 kg of crushed lithium fluoride in 100 liters of anhydrous tributylamine solvent;
[0045] (2) Filtrating the lithium fluoride solution to remove insoluble matter to obtain a filtrate;
[0046] (3) Pass 252 kg of phosphorus pentafluoride into the filtrate, and continue to heat and stir at 60°C for 1 hour to obtain a suspension containing the precipitated product;
[0047] (4) The suspension was filtered, and the obtained filter cake was washed with pentane, and vacuum-dried at 50° C. for 1 hour to obtain a lithium hexafluorophosphate product.
[0048] In summary, the method of the present invention can efficiently synthesize lithium hexafluorophosphate. At the same time, the method has few steps, is simple and has low raw material prices.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com