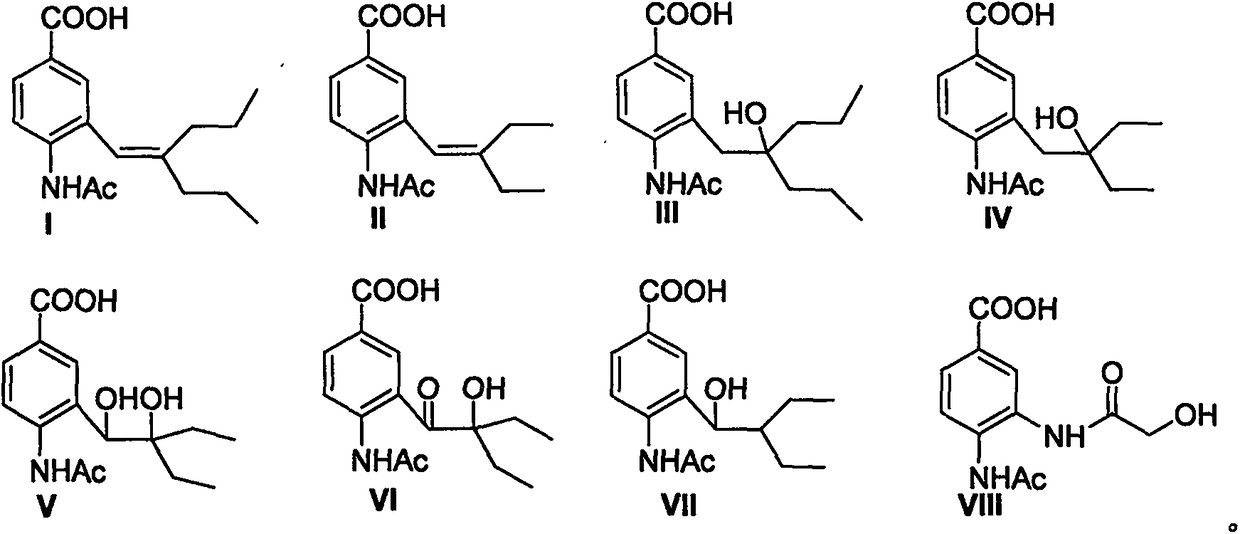
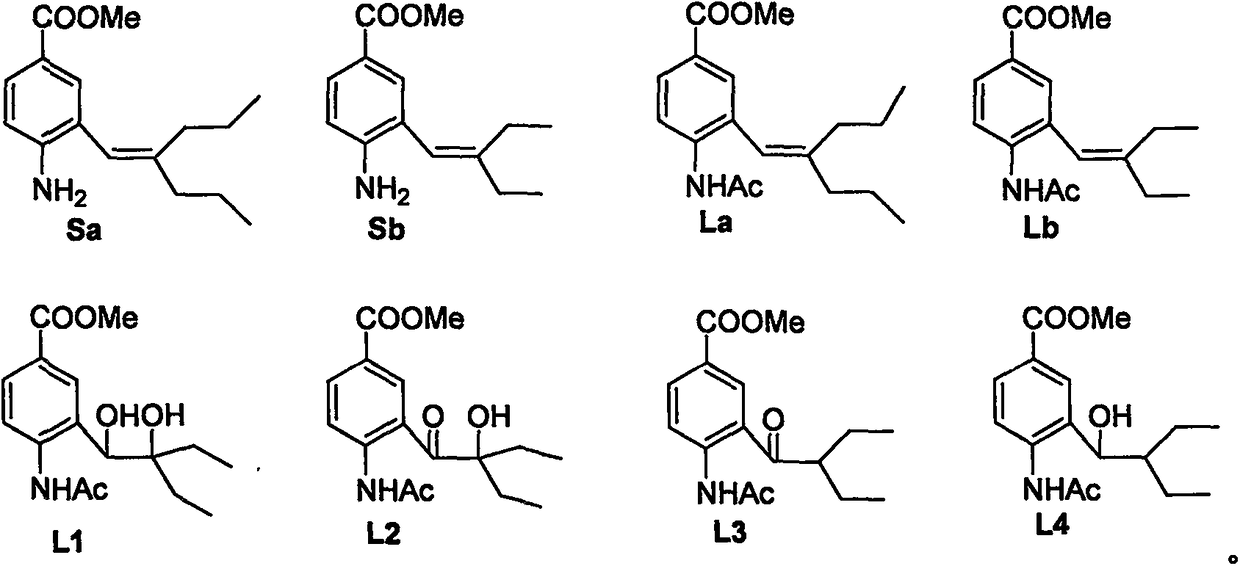
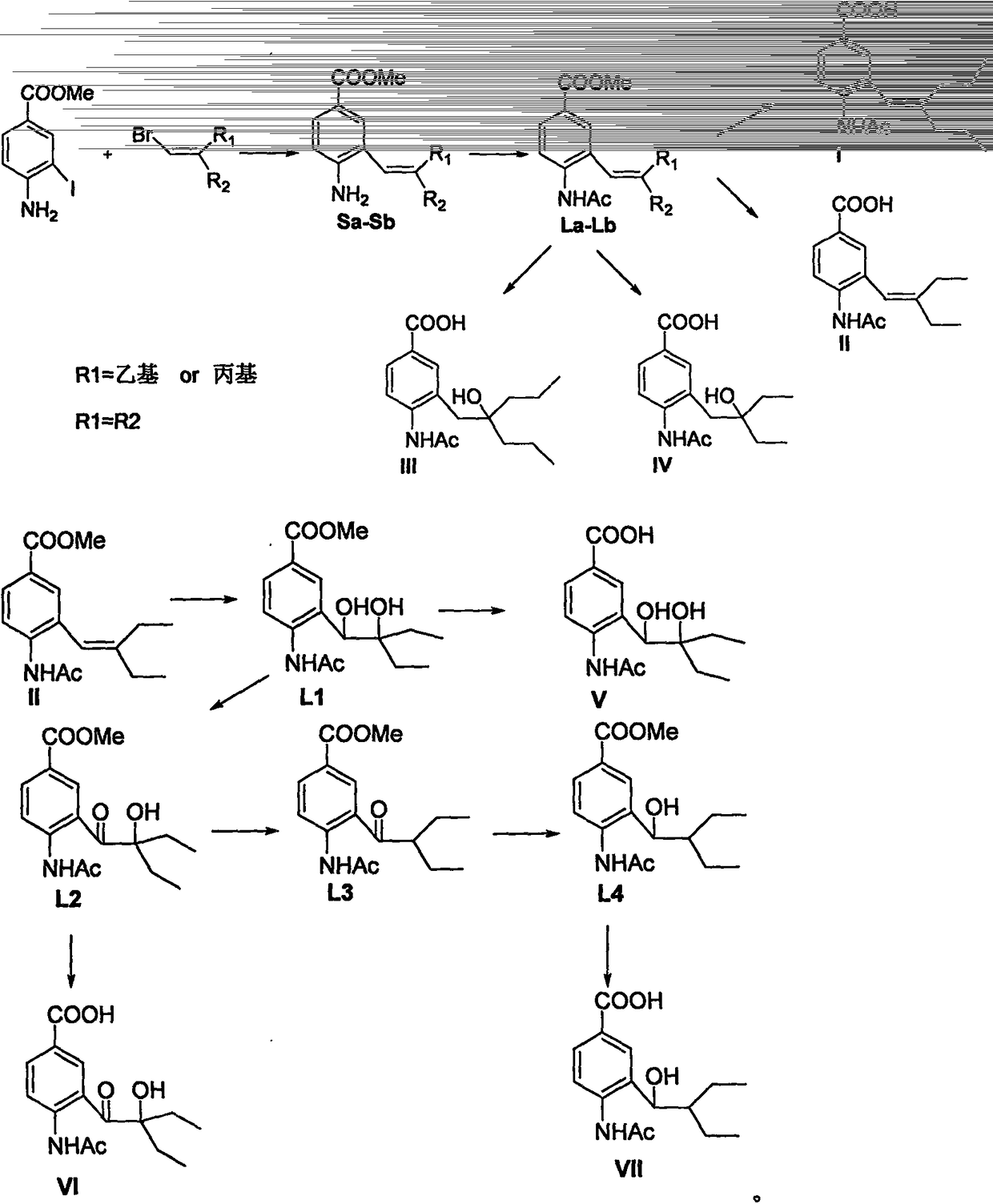
Anti-influenza virus compound and preparation method thereof
An anti-influenza drug and compound technology, applied in the field of medicine, can solve problems such as being unfavorable to the prevention and control of influenza outbreaks, unable to be equipped in primary medical institutions, increasing the economic burden of patients, etc., and achieving the effects of strong activity, low price and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation route of compound (La) is:
[0023]
[0024] The specific process is:
[0025] Preparation of 4-amino-3-(2-n-propyl-1-pentene)methyl benzoate (Sa): 1-bromo-3-n-propyl-1-pentene (6.7mmol) was dissolved in dioxygen Hexacyclic (30mL), stirred at room temperature under nitrogen protection, then added Et 3 N (3.7mL, 26.90mmol), Pd(PPh 3 ) 4 4-amino-3- Methyl iodobenzoate and Ba(OH) 2 ·8H 2 O (6.4g, 20.2mmol) was dissolved in 15ml of dioxane and added to the system, and the mixture was heated to 90°C and stirred for 8 hours. After the reaction solution was cooled down, it was filtered with diatomaceous earth, 40ml of saline was added to the filtrate, extracted with ethyl acetate (3×50mL), dried with anhydrous magnesium sulfate, filtered, concentrated, separated and purified by silica gel column chromatography to obtain Sa (free Colored oil, 76% yield): 1 H NMR (400MHz, CDCl 3 )δ0.81(t, J=7.4Hz, 3H), 0.97(t, J=7.4Hz, 3H), 1.40(m, 2H), 1.54(m 2H), 2....
Embodiment 2
[0028] The preparation route of compound (Lb) is:
[0029]
[0030] The specific process is:
[0031]Preparation of methyl 4-amino-3-(2-ethyl-1-butene)benzoate (Sb): 1-bromo-2-ethyl-1-butene (6.7mmol) was dissolved in dioxane ring (30mL), stirred at room temperature under nitrogen protection, then added Et 3 N (3.7mL, 26.90mmol), Pd(PPh 3 ) 4 4-amino-3- Methyl iodobenzoate and Ba(OH) 2 ·8H 2 O (6.4g, 20.2mmol) was dissolved in 15ml of dioxane and added to the system, and the mixture was heated to 90°C and stirred for 8 hours. After the reaction solution was cooled down, it was filtered with diatomaceous earth, 40ml of saline was added to the filtrate, extracted with ethyl acetate (3×50mL), dried with anhydrous magnesium sulfate, filtered, concentrated, separated and purified by silica gel column chromatography to obtain Sb (free Colored oil, 73% yield): 1 H NMR (400MHz, CDCl 3 )δ0.97(t, J=7.6, 3H), 1.14(t, J=7.4Hz, 3H), 2.08(q, J=7.4Hz, 2H), 2.23(q, J=7.6Hz, 2H), ...
Embodiment 3
[0034] The preparation route of compound (I) is:
[0035]
[0036] The specific process is:
[0037] Preparation of 4-acetamido-3-(2-n-propyl-1-pentene)benzoic acid (I): Dissolve La (61mg, 0.2mmol) in 2ml methanol, add 2ml 1Mol / L sodium hydroxide . The mixture was stirred at room temperature for 2-3 hours, and the pH of the solution was adjusted to 2 with 1Mol / L hydrochloric acid. After the mixture was evaporated to dryness, the solid residue was dissolved with 3ml of methanol, filtered, and the filtrate was concentrated. The obtained residue was subjected to silica gel column chromatography to obtain I (white solid, yield 61%): m.p.110-111°C, 1 H NMR (400MHz, CDCl 3 )δ0.80(t, J=7.3Hz, 3H), 1.01(t, J=7.2Hz, 3H), 1.39(m, 2H), 1.59(m, 2H), 1.94(t, J=7.3, 2H ), 2.19(s, 3H), 2.24(t, J=7.3Hz, 2H), 6.09(s, 1H), 7.51(s, 1H), 7.83(s, 1H), 8.00(d, J=8.4Hz , 1H), 8.45 (d, J=8.4Hz, 1H); MS (ES) m / z 290 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com