Novel phosphorescent ruthenium compound as well as preparation method and application thereof
A ruthenium complex, a new technology, applied in the field of phosphorescent ruthenium complexes and its preparation, can solve the problems of aggravating hypoxic state, limiting therapeutic effect, insufficient oxygen supply, etc., and achieve the effect of guaranteed effect, simple process and abundant raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of auxiliary ligand
[0030]
[0031] Preparation of Compound 2: Compound 1 (180mg, 1mmol) and iodine oxide 41mg (1.3mmol) were stirred at room temperature, then 2.5mL of acetic acid was added, heated to reflux for 3h to obtain a purple-black solution, cooled to room temperature, added to deionized water to settle, room temperature Stand overnight, vacuum filter to obtain a yellow solid, then dissolve into chloroform to obtain a dark red solution, and then wash with saturated NaHCO 3 and saturated Na 2 S 2 o 3 Washed, the organic phase was dried with anhydrous sodium sulfate, and finally rotary evaporated to obtain dark brown solid compound 2, yield: 93%.
[0032] 1 H NMR (400MHz, CDCl 3 )8.89(dd, J=2Hz,1.6Hz,1H),8.69(dd,J=0.8Hz,0.4Hz,1H),8.41(dd,J=2Hz,2Hz,1H),8.20(dd,J=1.2 Hz,0.8Hz,1H), 7.83-7.78(m,1H),7.59(dt,J=1.2Hz,8.8Hz,1H),7.43(dd,J=4.8Hz,4.8Hz, 1H).
[0033] Preparation of Compound 4: Compound 2 (833mg, 0.8mmol), ammonium aceta...
Embodiment 2
[0037] Embodiment 2: the preparation of Ru1, Ru2 and Ru3
[0038]
[0039] Preparation of Compound Ru1: Compound 5 (100 mg), Compound 4 (92 mg), 1 mL of triethylamine, stirred with 3 mL of ethylene glycol at room temperature, reacted at 120 °C in the dark for 24 h, cooled to room temperature, added potassium hexafluorophosphate solution and stirred for 2 h After that, ethylene glycol and triethylamine were distilled off under reduced pressure, settled with ethyl acetate, purified by column chromatography, the polarity was dichloromethane: acetonitrile ratio of 10:1, and then recrystallized three times with dichloromethane and ether to obtain the complex Ru1, 26% yield.
[0040] 1 H NMR (400MHz, (CD 3 ) 2 SO)8.77(d,J=8Hz,1H),8.70-8.61(m,4H),8.58(d,J=8Hz,1H),8.15-8.09(m,3H),7.96-7.94(m,1H) ,7.92-7.90(m,1H),7.87-7.78(m,6H),7.74(dd,J=1.2Hz,5.2Hz,1H),7.61-7.58(m,1H),7.45(dd,J=2.6 Hz, 8.4Hz,1H),7.42-7.39(m,1H),7.37-7.33(m,4H),7.29-7.24(m,1H),7.22-7.18(m, 3H),7.12-7.09(m, 8H...
Embodiment 3
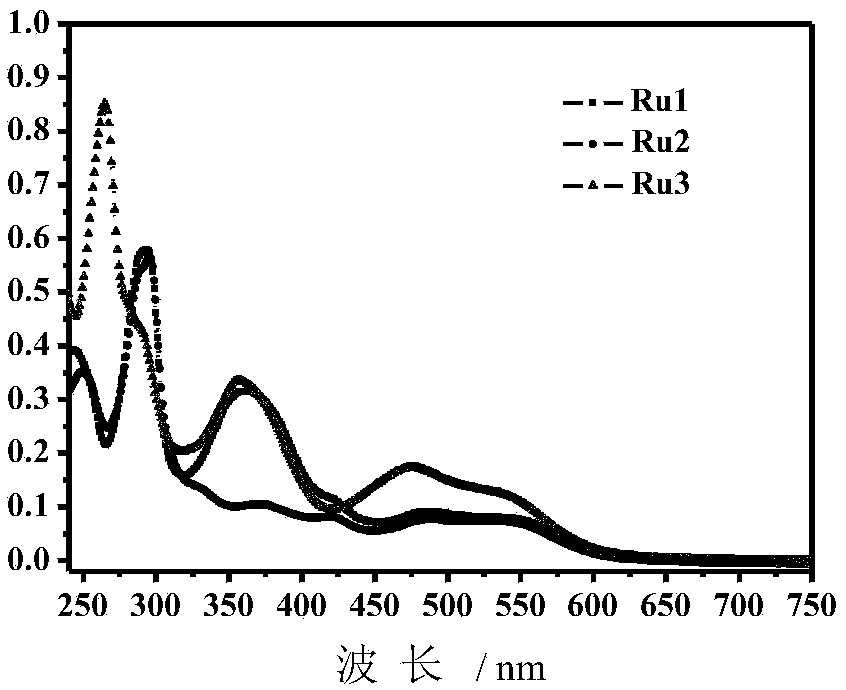
[0045] Embodiment 3: the ultraviolet-visible absorption spectrum test of Ru1, Ru2 and Ru3
[0046] The spectrum test concentration adopted in the present invention is 10 μM, and the test solvent is methanol solution.
[0047] Absorption spectra of Ru1, Ru2 and Ru3 as figure 1 As shown, 240-325nm is the ππ* transition centered on the N^N ligand, 325-425nm is the ππ* transition centered on the C^N ligand, and 425-650nm is the charge of the Ru center and the N^N ligand transfer (MLCT) transition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com