Micro-arc oxidation method in non-aqueous electrolyte system
A non-aqueous electrolyte, micro-arc oxidation technology, used in electrolytic coatings, anodizing, surface reaction electrolytic coatings, etc., to achieve excellent osteogenic activity, good adaptability and stability, and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
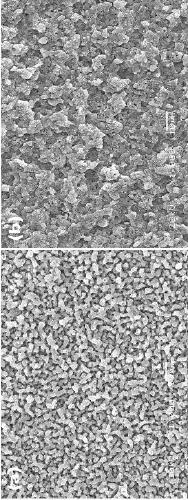
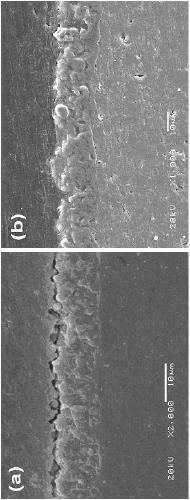
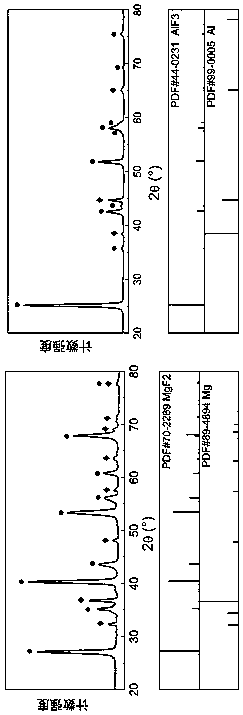
Image
Examples
Embodiment 1
[0027] Embodiment 1 A micro-arc oxidation method in a non-aqueous electrolyte system, comprising the following steps:
[0028] ⑴ Preparation of electrolyte:
[0029] Add 120 g of ammonium bifluoride to 1 L of mixed alcohol, stir at 65°C until completely dissolved, cool to room temperature, and then dissolve in 10 g of lactic acid to obtain an electrolyte.
[0030] Wherein: mixed alcohol refers to the mixed solution of 10 wt% absolute ethanol and 90 wt% ethylene glycol.
[0031] ⑵Sample pretreatment:
[0032] AZ91 magnesium alloy sample (25 mm×25 mm×10 mm), AM60 magnesium alloy sample (25 mm×25 mm×10 mm), WE43 magnesium alloy sample (25 mm×10 mm×5 mm), pure magnesium Sample (20 mm×10 mm×5 mm), 2024 aluminum alloy sample (25 mm×25 mm×5 mm), 6061 aluminum alloy sample (25 mm×25 mm×5 mm), ZL101 aluminum alloy sample (25 mm×25 mm×10 mm) and pure aluminum sample (20 mm×10 mm×5 mm) were polished and polished by 320#-600#-1000#-2000# water sandpaper respectively, and then passed th...
Embodiment 2
[0039] Embodiment 2 A micro-arc oxidation method in a non-aqueous electrolyte system, comprising the following steps:
[0040] ⑴ Preparation of electrolyte:
[0041] Add 120 g of ammonium fluoride to 1 L of mixed alcohol, stir at 50°C until completely dissolved, cool to room temperature, and then dissolve in 10 g of citric acid to obtain an electrolyte.
[0042] Wherein: mixed alcohol refers to the mixed solution of 10 wt% absolute ethanol and 90 wt% ethylene glycol.
[0043] ⑵Sample pretreatment:
[0044] After the AZ91 magnesium alloy sample (25 mm×25 mm×10 mm) and the 6061 aluminum alloy sample (25 mm×25 mm×5 mm) were polished by 320#-600#-1000#-2000# water sandpaper , rinsed with running water and deionized water, ultrasonically cleaned with absolute ethanol for 5 min, and then dried for later use.
[0045] ⑶ Micro-arc oxidation:
[0046] The pretreated sample is connected to the anode of the micro-arc oxidation power supply, and the stainless steel sheet is connected to...
Embodiment 3
[0051] Embodiment 3 A micro-arc oxidation method in a non-aqueous electrolyte system, comprising the following steps:
[0052] ⑴ Preparation of electrolyte:
[0053] Measure five parts of mixed alcohol, 1 L each, add 30 g of barium fluoride, cesium fluoride, lithium fluoride, ammonium fluoroborate, potassium fluoroborate respectively, stir at 40°C until completely dissolved, then cool to At room temperature, dissolve 6 g of lactic acid and 3 g of citric acid to obtain the electrolyte.
[0054] Wherein: mixed alcohol refers to the mixed solution of 10 wt% absolute ethanol and 90 wt% ethylene glycol.
[0055] (2) Sample pretreatment is the same as in Example 2.
[0056] ⑶ Micro-arc oxidation:
[0057] The pretreated sample is connected to the anode of the micro-arc oxidation power supply, and the stainless steel sheet is connected to the cathode, and placed in the electrolyte. The sample after the micro-arc oxidation treatment is rinsed with running water and deionized water,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com