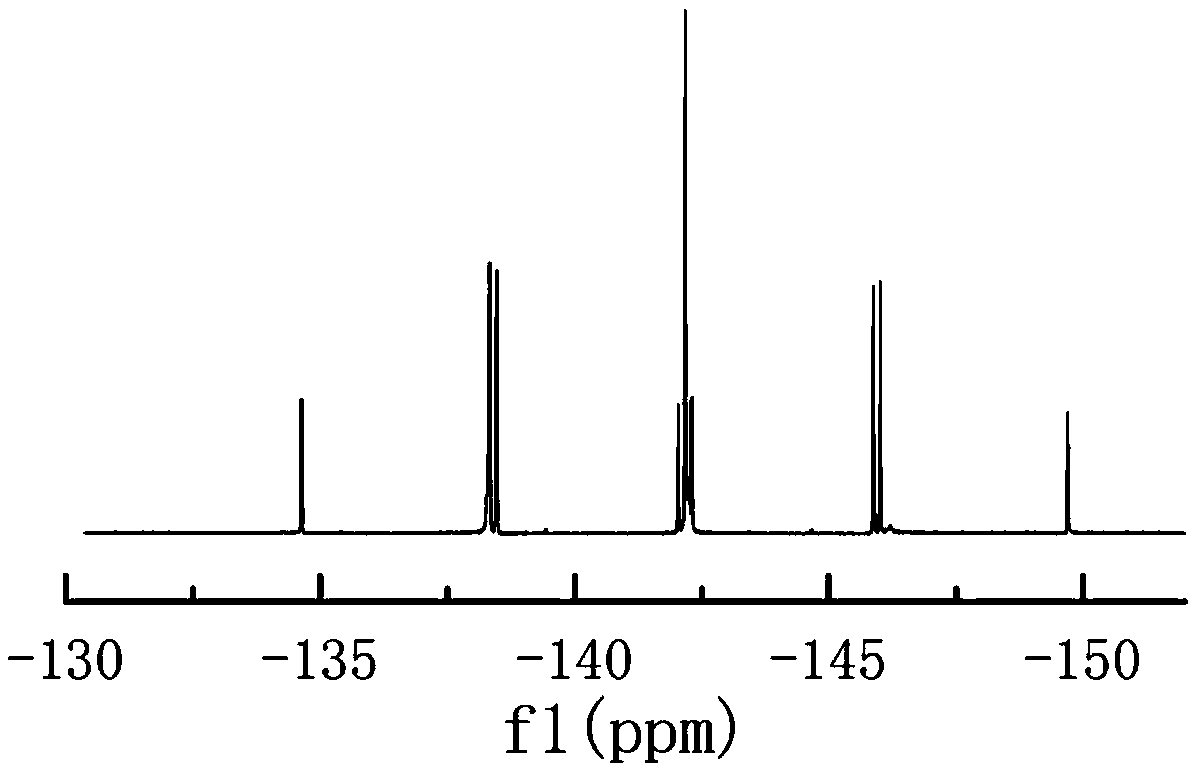
Preparation method of lithium tetrafluorooxalate phosphate
A technology of lithium tetrafluorooxalate phosphate and lithium hexafluorophosphate is applied in the field of lithium ion batteries, which can solve the problems of large reaction consumption, danger and complex reaction, and achieve the effects of short reaction time, low risk factor and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In a dry argon glove box, 228 g of dry ethyl methyl carbonate (EMC) was added to a three-necked flask, and lithium hexafluorophosphate (LiPF 6 ) 76.0g (0.5mol), dissolve, then drop into the flask 41.6g (0.2mol) of phosphorus pentachloride, one mouth of the flask is connected with a gas conduit with a valve, and the other two mouths are plugged with stoppers. The flask was taken out of the glove box, immersed in an ice-water bath and stirred. After half an hour, argon gas was slowly passed into the flask, and a stopper was opened at the same time, so that the inside of the flask was in an atmosphere protected by argon gas, and 45.0 g (0.5 mol) of anhydrous oxalic acid was quickly added to the flask, and then fully stirred. Slowly increase the temperature of the water bath to above 10°C, the reaction begins, and the generated gas flows into the solution containing sodium hydroxide for absorption. Continue to raise the temperature slowly until the solid dissolves, and the...
Embodiment 2
[0038] In a dry argon glove box, 304.0 g of dry dimethyl carbonate (DMC) was added to a three-necked flask, and lithium hexafluorophosphate (LiPF 6) 38.0g (0.25mol) for dissolution, then put 22.5g (0.25mol) of anhydrous oxalic acid into the flask, take the flask out of the glove box, immerse it in a water bath at 35°C and stir, and keep stirring the mixture. Slowly add 20.8 (0.1 mol) g of phosphorus pentachloride using a solid feeder, and the reaction begins to take place. The generated gas flows into a solution containing sodium hydroxide for absorption. It takes 1.5 hours to add phosphorus pentachloride, and then keep it at 35°C for one hour. No gas is emitted from the reaction, indicating that the reaction is complete. Under the protection of argon flow, 1800 mL of cyclohexane was added into the flask, and stirred slowly until crystals were precipitated. Lower the temperature of the water bath to -10°C, stop stirring, and after the crystals settle, filter, wash with cyclo...
Embodiment 3
[0041] In a dry argon glove box, 152.0 g of dry diethyl carbonate (DEC) was added to a three-necked flask, and lithium hexafluorophosphate (LiPF 6 ) 38.0g (0.25mol) for dissolution, then put 31.0g (0.25mol) of anhydrous ammonium oxalate into the flask, take the flask out of the glove box, immerse it in a water bath at 40°C and stir, and keep stirring the mixture. Phosphorus trichloride 22.9g (0.16mol) was slowly added dropwise using a constant pressure dropping funnel, and the reaction began to take place. The generated gas flows into a solution containing sodium hydroxide for absorption. It takes 1 hour to add phosphorus trichloride dropwise, and then keep the temperature at 40°C for 1 hour. If there is no gas released from the reaction, it means that the reaction is over. Under the protection of argon flow, 800 mL of dichloroethane was added to the flask, and stirred slowly until crystals were precipitated. Lower the temperature of the water bath to 0°C, stop stirring, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com