Preparation method of vonoprazan fumarate and intermediate thereof
A technology of vonoprazan fumarate and intermediates, which is applied in the field of medicinal chemistry and can solve the problems of many operation steps, cumbersome synthesis steps, and many impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
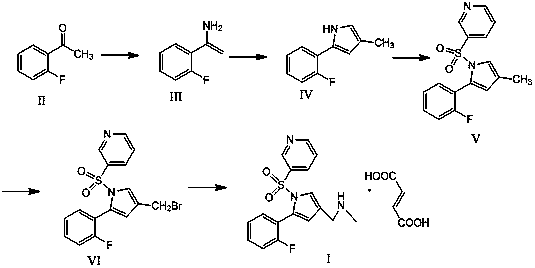
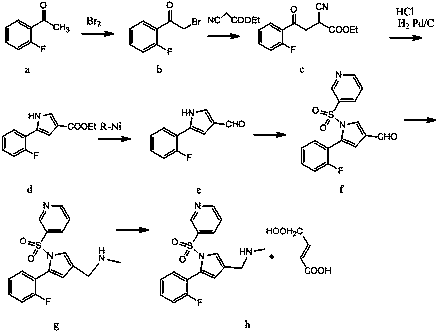
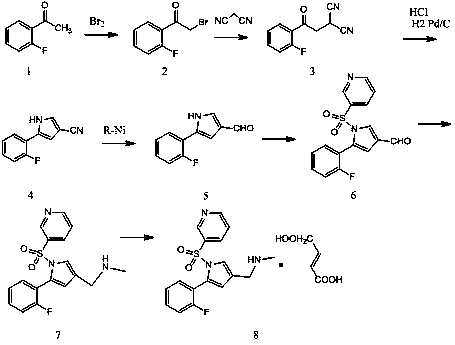
[0042] Preparation of Compound IV:
[0043] In the reaction flask, dissolve 100 g of the compound of formula II obtained by reacting formula I with sulfuric acid in nitrobenzene, add 120 g of 4-chlorobutane-2,3-dienoyl chloride, and under the action of catalyst aluminum tribromide, The compound of formula II and 4-chlorobutane-2,3-dienoyl chloride undergoes acylation reaction, and the molar ratio of the catalyst aluminum tribromide in the 4-chlorobutane-2,3-dienoyl chloride and the acylation reaction is 1:1.8, that is, add 126g of catalyst aluminum tribromide; the mass volume ratio of compound II and the solvent carbon disulfide in the acylation reaction is: 1g:6ml, that is, the solvent carbon disulfide is 600ml. 93.22 g of the compound of formula III was prepared by the sulfohydrolysis reaction, and the yield of the compound of formula III under this condition was 83.53%.
[0044] Dissolve 93.22 g of the prepared compound of formula III in toluene, put it into a reactor, add...
Embodiment 2
[0049] Preparation of Compound IV:
[0050] In the reaction flask, dissolve 100 g of the compound of formula II obtained by reacting formula I with sulfuric acid in nitrobenzene, add 120 g of 4-chlorobutane-2,3-dienoyl chloride, and under the action of catalyst aluminum tribromide, Formula II compound and 4-chlorobutane-2,3-dienoyl chloride undergo acylation reaction, the molar ratio of 4-chlorobutane-2,3-dienoyl chloride and the catalyst in the acylation reaction is 1:2.4, That is, 168g of aluminum tribromide is added as a catalyst; the mass volume ratio of compound II and the solvent in the acylation reaction is: 1g:8ml, that is, the solvent nitrobenzene is 800ml, and after reacting for 5 hours at a temperature of 28°C, the reaction is carried out by desulfonation and hydrolysis. 106.4 g of the compound of formula III was prepared, and the yield of the compound of formula III under the conditions was 92.38%.
[0051] Dissolve 106.4 g of the prepared compound of formula III ...
Embodiment 3
[0056] Preparation of compound VII:
[0057] Place 88.04 g of the prepared compound of formula IV in an inert gas steam, and at the reaction temperature, first pass a certain amount of ammonia water for 35 minutes, and pretreat the catalyst before the reaction to ensure that the water vapor and ammonia gas can be evenly distributed. Distributed in the catalyst aluminum silicate; then, furan is fed through another peristaltic pump, and ammonia water continues to be added dropwise at this time. The molar ratio of the compound of formula IV to ammonia and water was 1: (1:35), and 65.11 g of the compound of formula V was prepared, with a conversion rate of 82.99%.
[0058] Dissolve 65.11 g of the prepared compound of formula V in toluene, carry out sulfonamidation reaction with pyridine-3-sulfonyl chloride in a reactor, and prepare the compound of formula VI; dissolve the prepared compound of formula VI in ethanol, and undergo bromination After the reaction, the obtained substanc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com