Preparation of a ruthenium complex of terpyridine and its application in reverse transcriptase inhibition
A technology of ruthenium terpyridine and terpyridine, which is applied in the research and development field of HIV reverse transcriptase inhibitors, can solve the problems of lack of light absorption, fluorescence and other spectral properties, low water solubility, etc., and achieves good spectral properties, stable structure, good AIDS Effect of Viral Reverse Transcriptase Inhibitory Ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
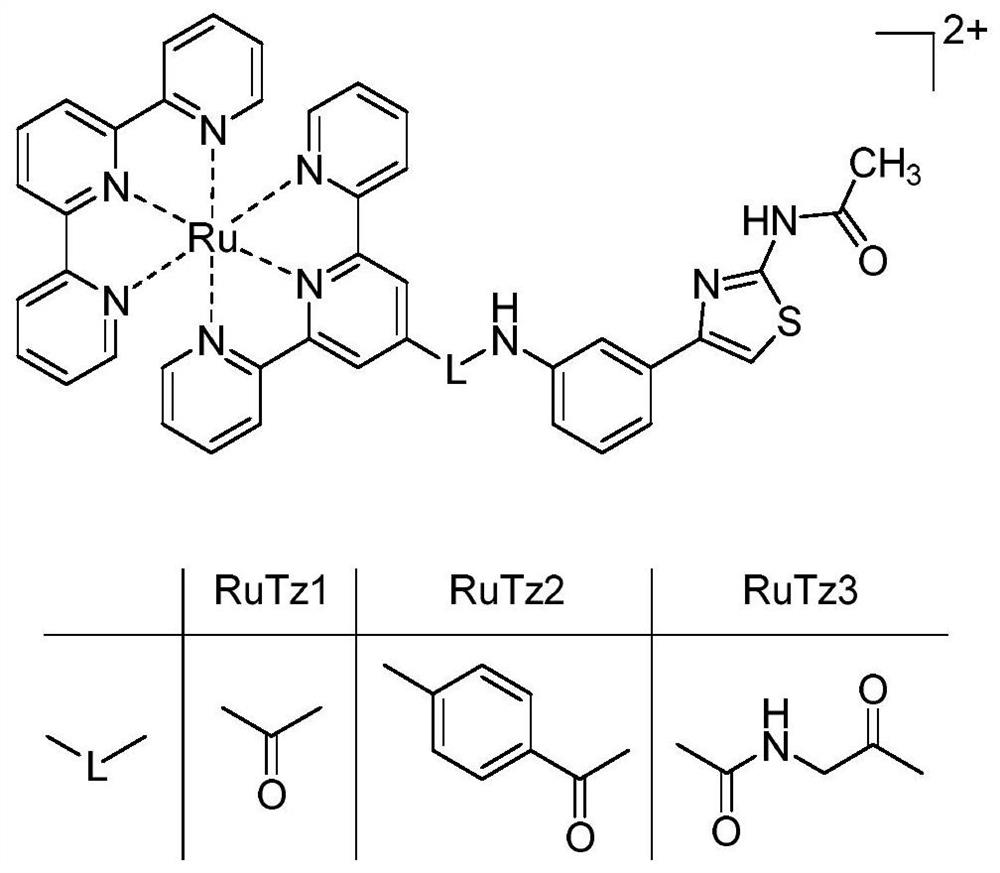
[0046] Example 1 Preparation of terpyridine ruthenium (II) complexes
[0047] The molecular structure of the synthesized terpyridine ruthenium (II) complex is as follows: figure 1 shown.
[0048] 1. Precursor complex [Ru(tpy)Cl 3 ] Preparation:
[0049] Precursor complex [Ru(tpy)Cl 3 ]according to figure 2 The indicated pathways were synthesized. Weigh 2-acetylpyridine (12.1 g, 0.1 mol) into a round bottom flask, add N,N-dimethylformamide dimethyl acetal (24.0 g, 0.2 mol) and 500 mL xylene, reflux for 4 hours . Xylene was distilled off under reduced pressure, and yellow crystals were obtained by recrystallization from n-pentane. The crystals were added to a solution of potassium tert-butoxide (23.0 g, 0.2 mol) and 2-acetylpyridine (12.1 g, 0.1 mol) in 500 mL of anhydrous tetrahydrofuran, and the reaction solution changed from bright yellow to pinkish yellow. Stir for 4 hours, add ammonium acetate (77.0 g, 1 mol) and acetic acid (250 mL), and stir for 5 minutes. All ...
Embodiment 2
[0058] Example 2 UV-visible detection of ruthenium(II) complexes interacting with RNA
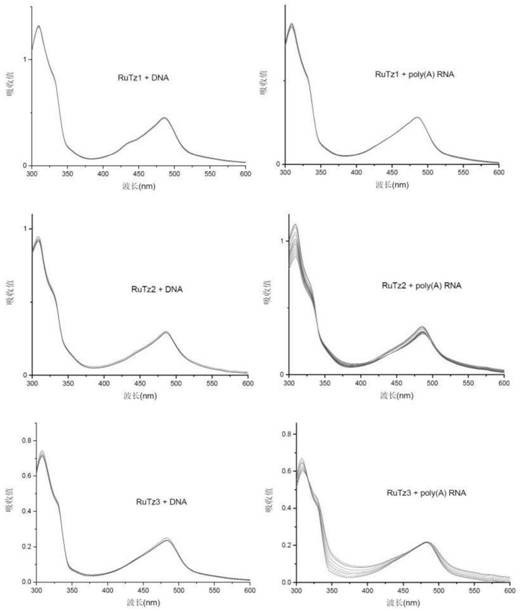
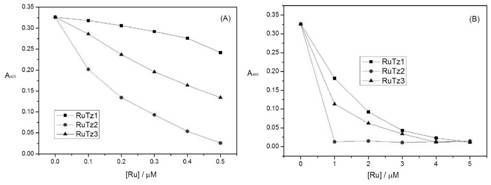
[0059]The configuration of the solution adopts the weighing method. The solvent is twice distilled water, and the buffer system is Tris-NaCl, pH 7.0. The concentration of the terpyridine ruthenium(II) complex is 2×10 -5 mol / L, poly(A) RNA and DNA concentration range is about 5×10 -6 ~5×10 -5 mol / L, the concentration of DNA or poly(A) RNA was gradually increased in the solution of fixed concentration of terpyridine ruthenium(II) complex, and the UV-vis spectra of the complex itself and different RNA concentrations were recorded respectively. Such as Figure 7 As shown, with the increase of DNA or poly(A) RNA concentration, the UV-visible spectrum of terpyridine ruthenium(II) complex RuTz1 basically did not change, while the UV-visible spectra of RuTz2 and RuTz3 increased with the addition of poly(A) RNA The change is obvious, but basically unchanged with the addition of DNA. This res...
Embodiment 3
[0060] Example 3 Recognition effect of terpyridine ruthenium (II) complex on HIV RNA
[0061] Gel electrophoresis was used to test the recognition effect of terpyridine ruthenium (II) complexes on the TAR region of HIV RNA. In a 0.2 mL PCR tube, prepare a series of 10 μL solutions containing 2×10 -6 mol / L TAR RNA, 2×10 -6 mol / L tat polypeptide, and 0~5×10 -5 mol / L terpyridine ruthenium (II) complex. The solution was incubated at 37°C for 30 minutes, 2 μL of RNA electrophoresis loading buffer was added, and electrophoresis was performed on a 10% polyacrylamide gel (denatured with urea) at 110 V for 1 hour. After staining with Gelred 4S nucleic acid dye for 15 minutes, take pictures with a gel imager and analyze the electrophoretic bands. Such as Figure 8 As shown, HIV TAR RNA itself shows a band. In the presence of tat polypeptide, part of the TAR RNA and the tat polypeptide are hydrogen bonded, so that electrophoresis shows two bands, that is, TAR RNA that is not bou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com