Rapid detection method of htlv-1/2 antibody
A detection method and antibody technology, applied in the field of medical detection, can solve the problems of high detection cost, difficult standardization of the process, false negative results, etc., and achieve the effect of simplifying the operation procedure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Selection of target genes and construction of expression strains:
[0052] Referring to the existing literature, it is determined to select gp46, gp21 of HTLV-1, gp46 and HTLV-1 / 2 of HTLV-2 (the above three genes are connected by Linker to form a chimeric gene) and a total of 4 genes are selected for experimental research. In order to obtain specific and immunologically active antigens for the establishment of serological methods for detecting HTLV antibodies. The specific antigenic epitope sequence is selected as follows: HTLV-1-gp46 162aa-109aa (Sequence ID: AAC35409.2), which is expressed as the gene of (162aa-109aa) amino acids on gp46 selected in HTLV-1 based on its sequence number It is AAC35409.2, and the following means HTLV-1-gp21 62aa-105aa (Sequence ID: AAA65883.1), HTLV-2-gp4631aa-74aa (Sequence ID: ADN52094.1). After determining the amino acid sequence of the antigenic epitope, retrieve the gene sequence from NCBI, and connect the three genes through Linke...
Embodiment 2
[0054] Induced expression, purification and activity identification of recombinant expression strains:
[0055] The overnight activated recombinant expression strain was transferred to LB liquid medium containing Amp+ resistance (Amp+, final concentration 100ug / mL) at a ratio of 1-1.5:100, cultured on a shaker at 37°C at 220rpm; cultured until assayed When the OD600 value of the bacterial solution is between 0.5-0.8, IPTG with a final concentration of 0.1mM is added to induce expression, and SDS-PAGE protein gel is used to detect whether the protein is expressed and the level of expression. Expand the culture volume according to the above conditions, harvest the bacteria, and bathe the cultured bacteria in ice for 10 minutes, then centrifuge at 4°C and 7000rpm for 10 minutes, collect the precipitate and discard the supernatant, and measure the wet weight of the precipitate, and use the bacteria liquid lysis buffer (other Contains: 50mM Phosphate Buffer pH 7.4 containing 0.5M N...
Embodiment 3
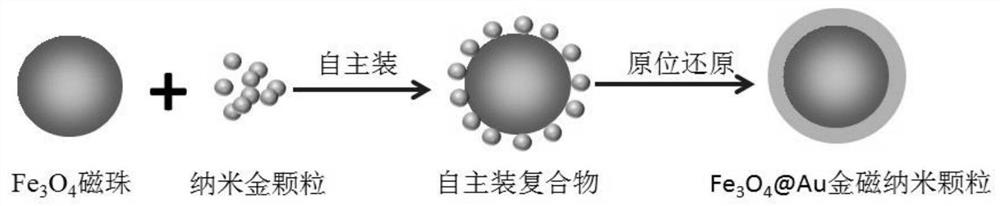
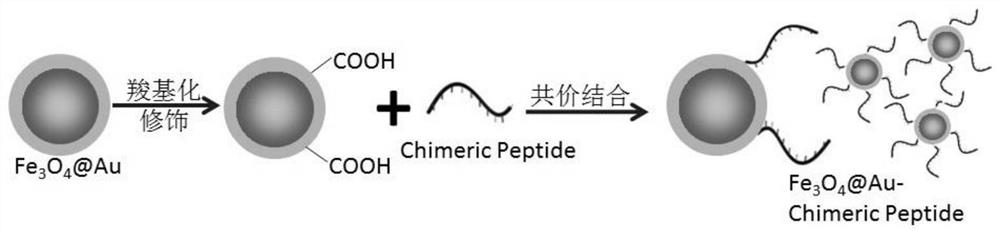
[0057] Preparation of gold magnetic nanospheres and functional modification:
[0058] (1) Ferrofluid preparation: In the case of passing nitrogen gas first, add 9.44g Fe to the round bottom flask 3+ , 4.13g Fe 2 + , 1M 160mL NaOH and 160mL water, stir to dissolve, 40°C, 500rpm, react for 20min, then raise the temperature to 60°C, increase the speed to 2000rpm, react for 20min to collect the magnetic fluid, wash for later use.
[0059] (2) Preparation of gold magnetic nanoparticles: take 120mg magnetic fluid, shake, 220rpm, 37°C, 30min, add 250mL of chloroauric acid with a mass fraction of 0.025%, 220rpm, 37°C, 40min, make Au 3+ Fully adsorbed on the surface of the seed microspheres, then add an excess of 80mmol / l hydroxylamine hydrochloride as a reducing agent, 220rpm, 37°C, 30min, then add 50mL of chloroauric acid with a mass fraction of 0.05%, 220rpm, 37°C, 30mim, that is Form Fe 3 o 4 @Au gold magnetic nanoparticles, finish the reaction, wash with purified water until ne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com