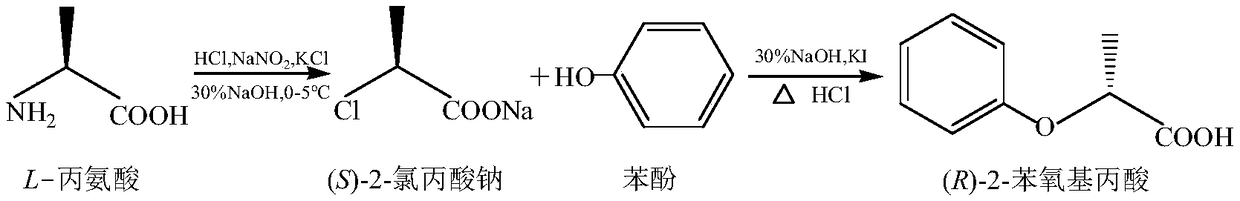
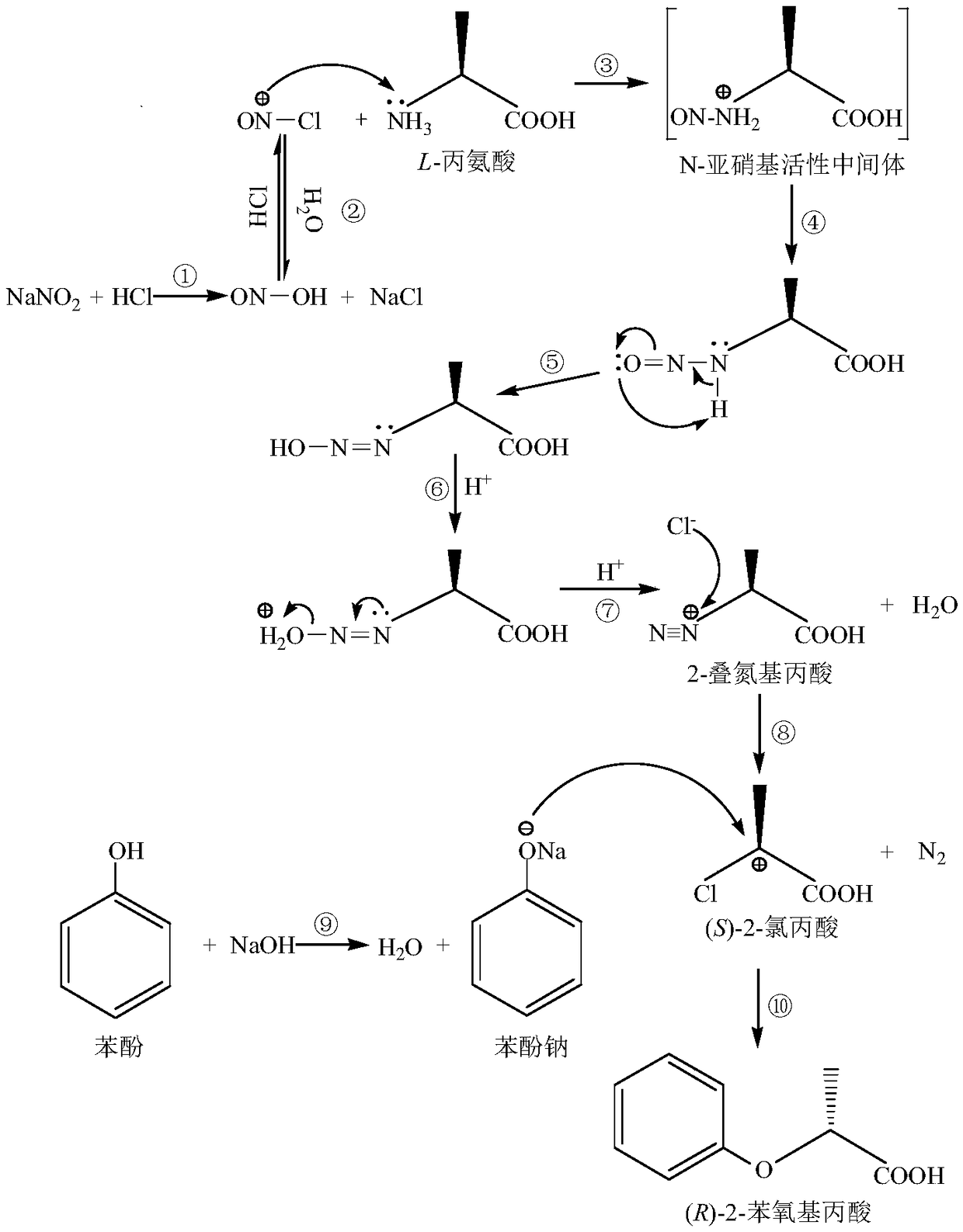
Chemical synthesis method of (R)-2-phenoxypropionic acid
A phenoxy propionic acid, chemical synthesis technology, applied in chemical instruments and methods, organic chemistry, carboxylate preparation, etc., can solve the problems of large environmental pollution, many by-products, high energy consumption, etc., and achieve high product purity, The effect of easy to obtain raw materials and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Reagents and instruments
[0038] Instruments: Waters 2695 high-performance liquid chromatography; Autopol IV-T polarimeter; Bruker AVANCEIII 500MHz nuclear magnetic resonance spectrometer; Nicolet 6700 Fourier transform infrared spectrometer.
[0039] Reagents: L-alanine (AR), TCI (Shanghai) Chemical Industry Development Co., Ltd.; phenol (AR), Sinopharm Chemical Reagent Co., Ltd.; sodium carbonate (AR), Taicang Meida Reagent Co., Ltd.; chloride Potassium (AR), Sinopharm Chemical Reagent Co., Ltd.; potassium iodide (AR), Sinopharm Chemical Reagent Co., Ltd.; hydrochloric acid (36-38%, AR), Xilong Science Co., Ltd.; sodium nitrite (AR), Guangdong Guanghua Technology Co., Ltd.; Sodium Hydroxide (AR), Xilong Science Co., Ltd.
[0040] 2. Drawing of (S)-2-chloropropionic acid gas chromatography detection standard curve
[0041] Gas chromatography column: capillary column (30m×0.25mm); detector: FID; N 2 Flow rate: 30mL / min; H 2 Flow rate: 40mL / min; O 2 Flow rate: 4...
Embodiment 2
[0061] Embodiment 2 (S)-2-chloropropionic acid synthesis condition optimization
[0062] 1. Optimization of the molar ratio of L-alanine to hydrochloric acid
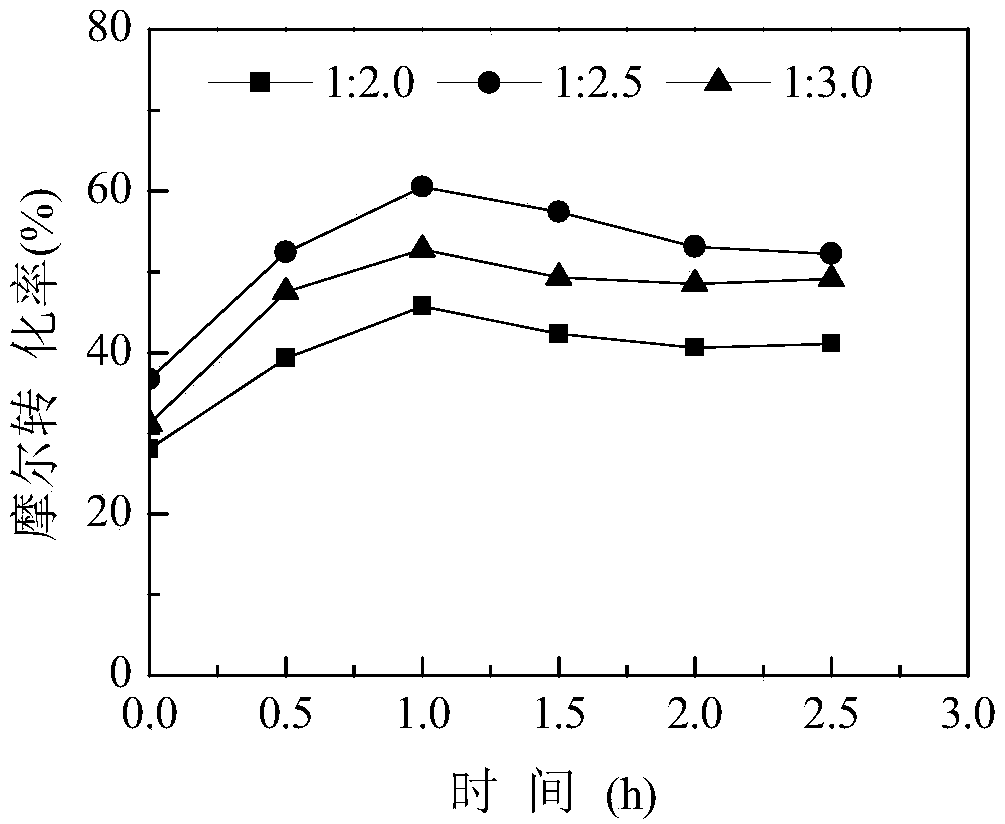
[0063] The experimental operation is the same as in Example 1, and the molar ratio of the step (1) L-alanine to hydrochloric acid in Example 1 is changed to 1:2, 1:2.5 and 1:3. After the sodium nitrite is added dropwise, react 2.5 h, sampling at intervals of 0.5h, the conversion rate of L-alanine is detected by gas phase, and the influence of the molar ratio of L-alanine and hydrochloric acid on the synthesis of (S)-2-chloropropionic acid is investigated, the results are as follows image 3 , so as to select the optimal hydrochloric acid dosage.
[0064] Depend on image 3 As can be seen, increasing the amount of hydrochloric acid is beneficial to the synthesis of (S)-2-chloropropionic acid, and when L-alanine: hydrochloric acid is 1:2.5 (mol / mol), the (S)-2-chloropropionic acid The molar conversion rate is the highe...
Embodiment 3
[0071] Embodiment 3 (R)-PPA synthesis condition optimization
[0072] 1. Optimization of etherification reaction temperature
[0073] The experimental operation is the same as that in Example 1, and the etherification reaction temperature in Step (2) of Example 1 is changed to 25, 45, 65, 85 and 125° C., respectively reacting for 3 hours, sampling at intervals of 0.5 hours, and detecting the conversion rate of the substrate by HPLC. Investigate the influence of etherification reaction temperature on (R)-2-phenoxypropionic acid synthesis, the results are as follows Figure 6 , so as to select the optimal reaction temperature.
[0074] Depend on Figure 6 It can be seen that the temperature rise is beneficial to the synthesis of (R)-2-phenoxypropionic acid, and when it reaches reflux at 125 ° C, the molar conversion rate of (R)-2-phenoxypropionic acid at different reaction times is higher than that of other temperature. This may be because on the one hand, reflux can increas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com