ALT (atenolol) hapten, artificial antigen, antibody, preparation methods of ALT hapten and artificial antigen and applications of hapten and antibody
An artificial antigen and hapten technology, applied in chemical instruments and methods, preparation of carboxylic acid amides, preparation of organic compounds, etc., can solve the problems of cumbersome processing, long detection time, expensive instruments, etc., and achieve low cross-reaction rate and affinity. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Hapten synthesis
[0025] A kind of atenolol hapten, the molecular structure is:
[0026]
[0027] It is used as a raw material for the antigen system of animal immunity.
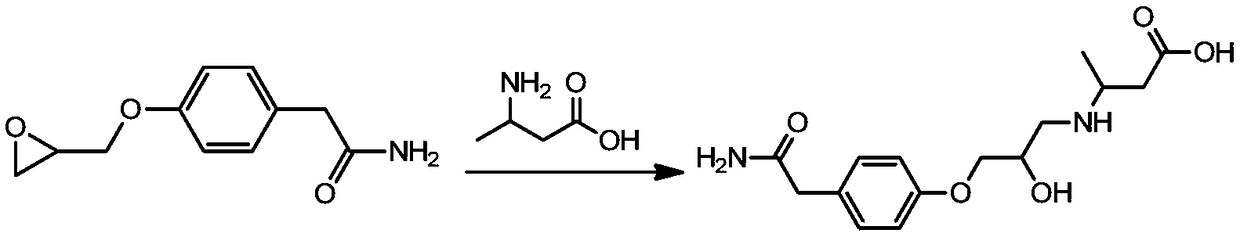
[0028] The preparation method of the above-mentioned atenolol hapten is as follows (see the attached synthetic route figure 1 ):
[0029] Take 0.5 g of 4-(epoxyethyl methoxy) phenylacetamide, add 60 mL of ethanol to dissolve, add 0.5 mL of 1 mol / L hydrochloric acid, stir and mix, add 0.29 g of L-β-homoalanine hydrochloride, Heat and stir in an oil bath, react at 80°C for 4 hours; stop the reaction, rotary steam, remove ethanol, add 80 mL of water, add 100 mL of chloroform, extract three times, combine the organic phases, evaporate to dryness, and purify by silica gel column chromatography. The volume ratio is The atenolol hapten can be obtained by eluting with 10:1 dichloromethane-methanol.
Embodiment 2
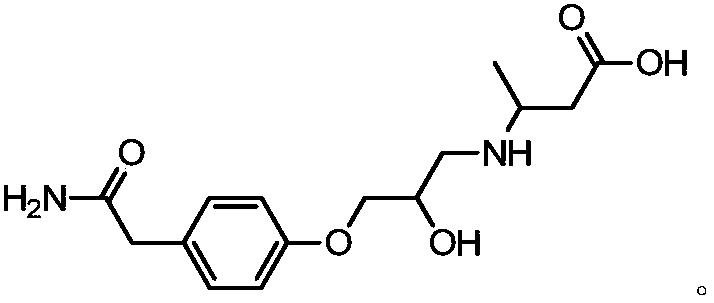
[0030] Example 2: Artificial antigen synthesis and identification
[0031] An artificial antigen of Atenolol made from the hapten of Atenolol described in Example 1. The molecular structure is:
[0032]
[0033] 1. Synthesis of immunogen
[0034] The synthesis method of immunogen is as follows:
[0035] Take 11mg of atenolol hapten, add 2mL N,N-dimethylformamide (DMF) to dissolve, add 8mg carbodiimide (EDC), 7mg N-hydroxysuccinimide (NHS), stir at room temperature for 2h , Get hapten activation solution A; take 50mg bovine serum albumin (BSA), add 3mL of 0.1mol / L phosphate buffer to dissolve to get B solution; add drop A to B solution, stir 4h, 0.02mol / L PB buffer solution was purified by dialysis for 3 days, and the medium was changed 3 times a day to obtain atenolol immunogen, aliquoted, and stored at -20°C.
[0036] Identification of artificial antigens:
[0037] According to the ratio of hapten, carrier protein and coupling product used in the synthesis of atenolol immunogen react...
Embodiment 3
[0042] Example 3: Monoclonal antibody preparation
[0043] A specific antibody of atenolol, which is obtained by immunizing white mice with the atenolol immunogen described in Example 2 and can react specifically with atenolol for monoclonal immunoglobulin G for Detection of atenolol residues.
[0044] The preparation method of the above-mentioned atenolol monoclonal antibody is as follows:
[0045] 1) Animal immunization: The immunogen was injected into Balb / c mice, and the immunization dose was 150 μg / mouse to produce antiserum.
[0046] 2) Cell fusion and cloning: The spleen cells of Balb / c mice that produce specific antibodies are fused with myeloma cells SP20, and the cell supernatant is determined by indirect competitive enzyme-linked immunoassay to screen positive wells. The positive wells were cloned by the limiting dilution method to obtain and establish a hybridoma cell line producing monoclonal antibodies.
[0047] 3) Cell cryopreservation and resuscitation: The hybridoma c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com