Method for preparing lanthanum metal by low-temperature electrodeposition by taking lanthanum chloride as raw material
A technology of lanthanum chloride and metal lanthanum, which is applied in the improvement of process efficiency, photographic technology, instruments, etc., can solve the possibility of increasing the production cost of ionic liquids and environmental pollution, has no large-scale practical application background, and restricts the practical application of ionic liquids. and other problems, to achieve the effects of good chemical and thermal stability, excellent solubility, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
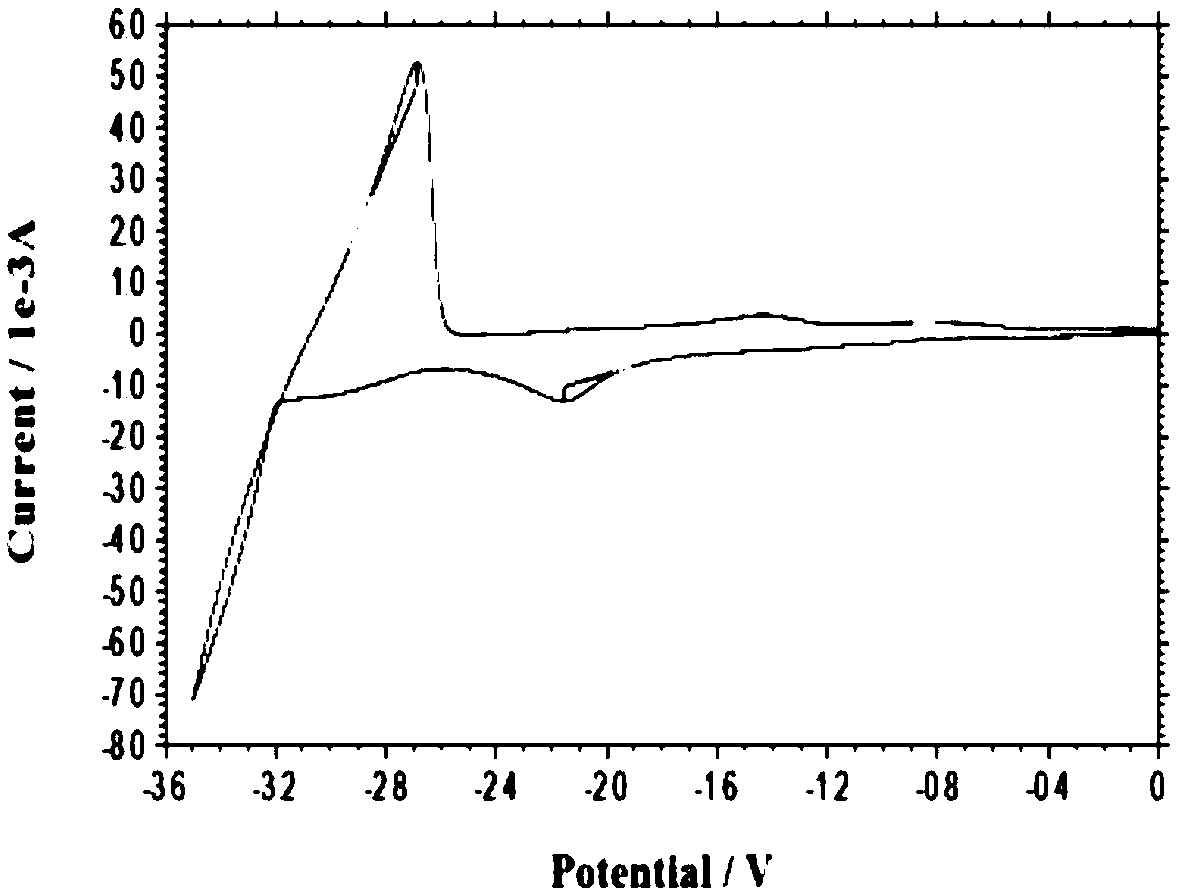
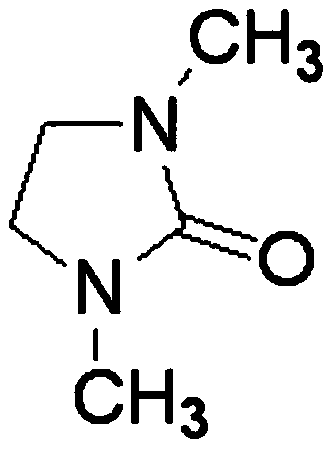
[0035] The electrolyte raw materials are prepared as lanthanum chloride and lithium nitrate, and the solvent DMI is stirred and mixed in the electrolytic cell to form an electrolyte system, wherein the molar concentrations of lanthanum chloride and lithium nitrate are 0.01mol / L and 0.1mol / L, respectively. Control the constant temperature of the electrolyte system at 25°C, the electrolysis voltage is -2.0V (vs Ag), and the anode material is graphite; after 30 minutes of electrolysis, add lanthanum chloride once to make the concentration of lanthanum chloride in the system 0.01mol / L; after 60 minutes of electrolysis, the substrate The sediments on the site are collected and preserved, and the collected sediments are reprocessed according to the needs. After characterization and detection, the results show that metal lanthanum can be effectively deposited, and the total content of lanthanum element detected by ICP is 92.82%.
Embodiment 2
[0037] The electrolyte raw materials are prepared as lanthanum chloride and lithium nitrate, solvent DMI, and stirred and mixed in the electrolytic cell to form an electrolyte system, wherein the molar concentrations of lanthanum chloride and lithium nitrate are 0.02mol / L and 0.2mol / L, respectively. Control the constant temperature of the electrolyte system at 35°C, the electrolysis voltage is -2.1V (vs Ag), and the anode material is a tungsten rod; after 30 minutes of electrolysis, add lanthanum chloride once to make the concentration of lanthanum chloride in the system 0.03mol / L; after 60 minutes of electrolysis, add The deposits on the substrate are collected and preserved, and the collected deposits are reprocessed as required. After characterization and detection, the results show that metal lanthanum can be effectively deposited, and the total content of lanthanum element detected by ICP is 98.76%.
Embodiment 3
[0039]The electrolyte raw materials are prepared as lanthanum chloride and lithium nitrate, and the solvent DMI is stirred and mixed in the electrolytic cell to form an electrolyte system, wherein the molar concentrations of lanthanum chloride and lithium nitrate are 0.05mol / L and 0.5mol / L, respectively. Control the constant temperature of the electrolyte system at 45°C, the electrolysis voltage is -2.2V (vs Ag), and the anode material is a molybdenum rod; after 30 minutes of electrolysis, add lanthanum chloride once to make the concentration of lanthanum chloride in the system 0.05mol / L; after 60 minutes of electrolysis, add The deposits on the substrate are collected and preserved, and the collected deposits are reprocessed as required. After characterization and detection, the results show that metal lanthanum can be effectively deposited, and the total content of lanthanum element detected by ICP is 98.84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com