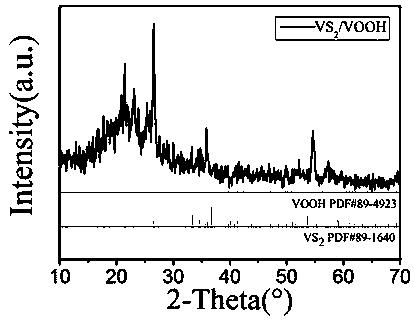
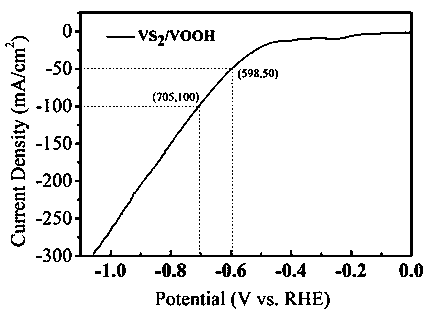
Nanometer floriform vanadium disulfide/hydroxy vanadium oxide difunctional composite electrocatalyst and preparation method thereof
A vanadium disulfide, hydroxyl oxidation technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of electrochemical application limitations, high cost, etc., and achieve a simple synthesis route, low synthesis Uniform effect of temperature and chemical composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Soak the graphite paper in pure acetone solution for 5 minutes, then immerse the graphite paper in 3mol / L hydrochloric acid for 5 minutes, and finally wash it with ethanol and deionized water alternately for 3 times, vacuum at 25°C Obtain the treated graphite paper after drying for 15h;
[0021] (2) Weigh NH 4 VO 3 and C 2 h 5 Add NS to 20ml deionized water at the same time to control NH 4 VO 3 :C 2 h 5 The molar ratio of NS is 1:10, and 1ml of ammonia water is added. At this time, the concentration of the vanadium source is 0.05mol / L, and the concentration of the sulfur source is 0.5mol / L. Stir magnetically at room temperature for 30min to obtain a clear solution A;
[0022] (3) Put the graphite paper processed in step (1) into the reaction liner, then pour solution A into the reaction liner and seal it, then install the liner in the outer kettle and fix it in a homogeneous reactor , then reacted at 160°C for 18h;
[0023] (4) After the hydrothermal reacti...
Embodiment 2
[0025] (1) Soak the graphite paper in pure acetone solution for 10 minutes, then immerse the graphite paper in 2mol / L hydrochloric acid for 10 minutes, and finally rinse it with ethanol and deionized water for 4 times, and vacuum at 35°C Obtain the treated graphite paper after drying for 14h;
[0026] (2) Weigh NH 4 VO 3 and C 2 h 5 Add NS to 25ml deionized water at the same time to control NH 4 VO 3 :C 2 h 5 The molar ratio of NS is 1:6, and 2ml of ammonia water is added. At this time, the concentration of the vanadium source is 0.08mol / L, and the concentration of the sulfur source is 0.48mol / L. Stir magnetically at room temperature for 40min to obtain a clear solution A;
[0027] (3) Put the graphite paper processed in step (1) into the reaction liner, then pour solution A into the reaction liner and seal it, then install the liner in the outer kettle and fix it in a homogeneous reactor , then reacted at 180°C for 20h;
[0028] (4) After the hydrothermal reaction is...
Embodiment 3
[0030] (1) Soak the graphite paper in pure acetone solution for 15 minutes, then immerse the graphite paper in 4mol / L hydrochloric acid for 10 minutes, and finally wash it with ethanol and deionized water alternately for 3 times, vacuum at 40°C Obtain the treated graphite paper after drying for 12h;
[0031] (2) Weigh NH 4 VO 3 and C 2 h 5 Add NS to 30ml deionized water at the same time to control NH 4 VO 3 :C 2 h 5 The molar ratio of NS is 3:14, and 3ml of ammonia water is added. At this time, the concentration of the vanadium source is 0.1mol / L, and the concentration of the sulfur source is 0.467mol / L. Stir magnetically at room temperature for 45min to obtain a clear solution A;
[0032] (3) Put the graphite paper processed in step (1) into the reaction liner, then pour solution A into the reaction liner and seal it, then install the liner in the outer kettle and fix it in a homogeneous reactor , then reacted at 180°C for 22h;
[0033] (4) After the hydrothermal rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com