Composite photocatalyst and application thereof
A photocatalyst and catalyst technology, applied in the direction of physical/chemical process catalysts, heterogeneous catalyst chemical elements, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problems of low ammonia production rate, unrealized application, increase Response costs and other issues, to achieve the effect of simple raw materials, mild construction, simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1MgO-CoO x / TiO 2 Preparation of Synthetic Ammonia Photocatalyst
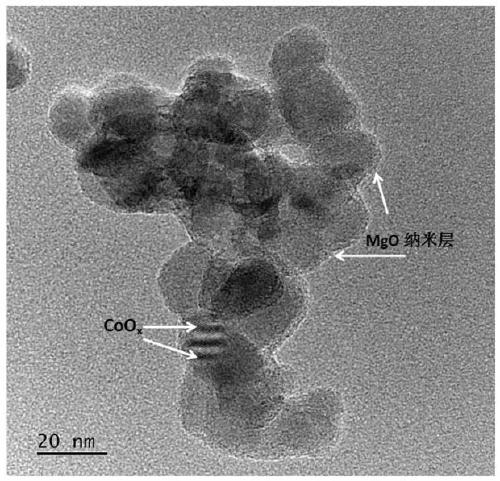
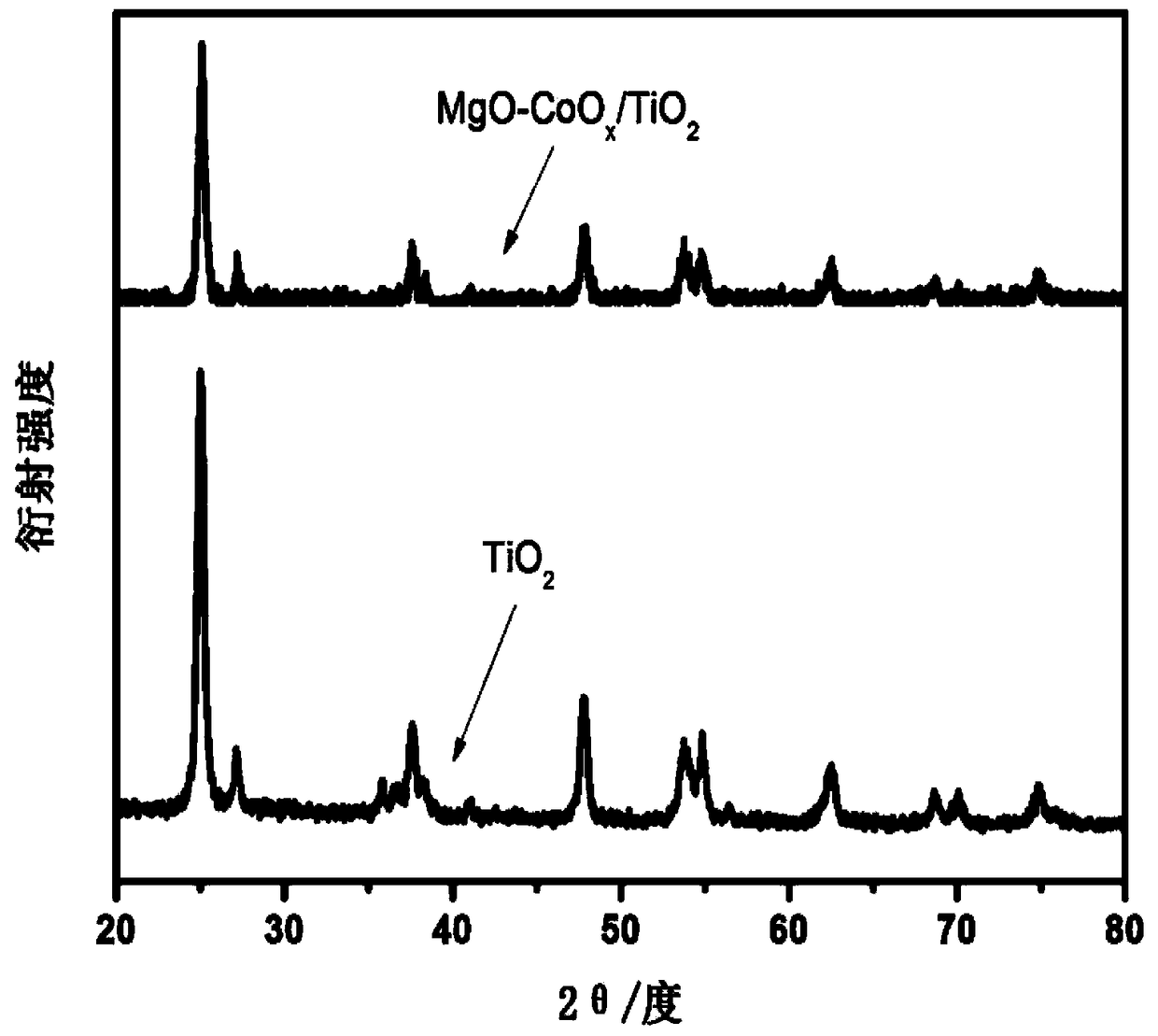
[0038] The experimental steps include: 1. First weigh 0.5g of nano-TiO 2 Powder, add to 50ml of water and keep stirring. 2. Add 2 mmol of magnesium nitrate, and raise the temperature to 100°C under continuous stirring until the liquid evaporates completely. 3. Take out the dried powder and place it in a muffle furnace, raise the temperature to 350°C, and cool down to achieve the loading of the MgO co-catalyst. Then filter cake is obtained by filtration. 5. Put the filtered powder in the muffle furnace again, raise the temperature to 300°C, and cool down to achieve CoO x Cocatalyst loading. The obtained sample is denoted as MgO-CoO x / TiO 2 . figure 1 For the X-ray powder diffraction pattern (XRD) of synthetic sample, by figure 1 It can be seen that the main phase of the synthesized sample is TiO 2 , image 3 For the transmission electron microscope (TEM) of the synthesized sample, M...
Embodiment 2
[0039] Example 2MgO-CoO x / g -C 3 N 4 Preparation of Synthetic Ammonia Photocatalyst
[0040] Experimental procedure comprises: 1. at first take by weighing 5g melamine and 0.1g magnesium nitrate, both are mixed. 2. Heat the obtained mixture in a muffle furnace to 550° C., and cool down. 3. Disperse the obtained powder in 1 mmol of cobalt nitrate aqueous solution and impregnate it, and then obtain a filter cake by filtration. 4. Put the filtered powder in the muffle furnace again and keep it warm at 300°C to realize CoO x Cocatalyst loading. The obtained sample is denoted as MgO-CoO x / g -C 3 N 4 .
Embodiment 3
[0041] The operating method of embodiment 3 composite photocatalyst synthetic ammonia
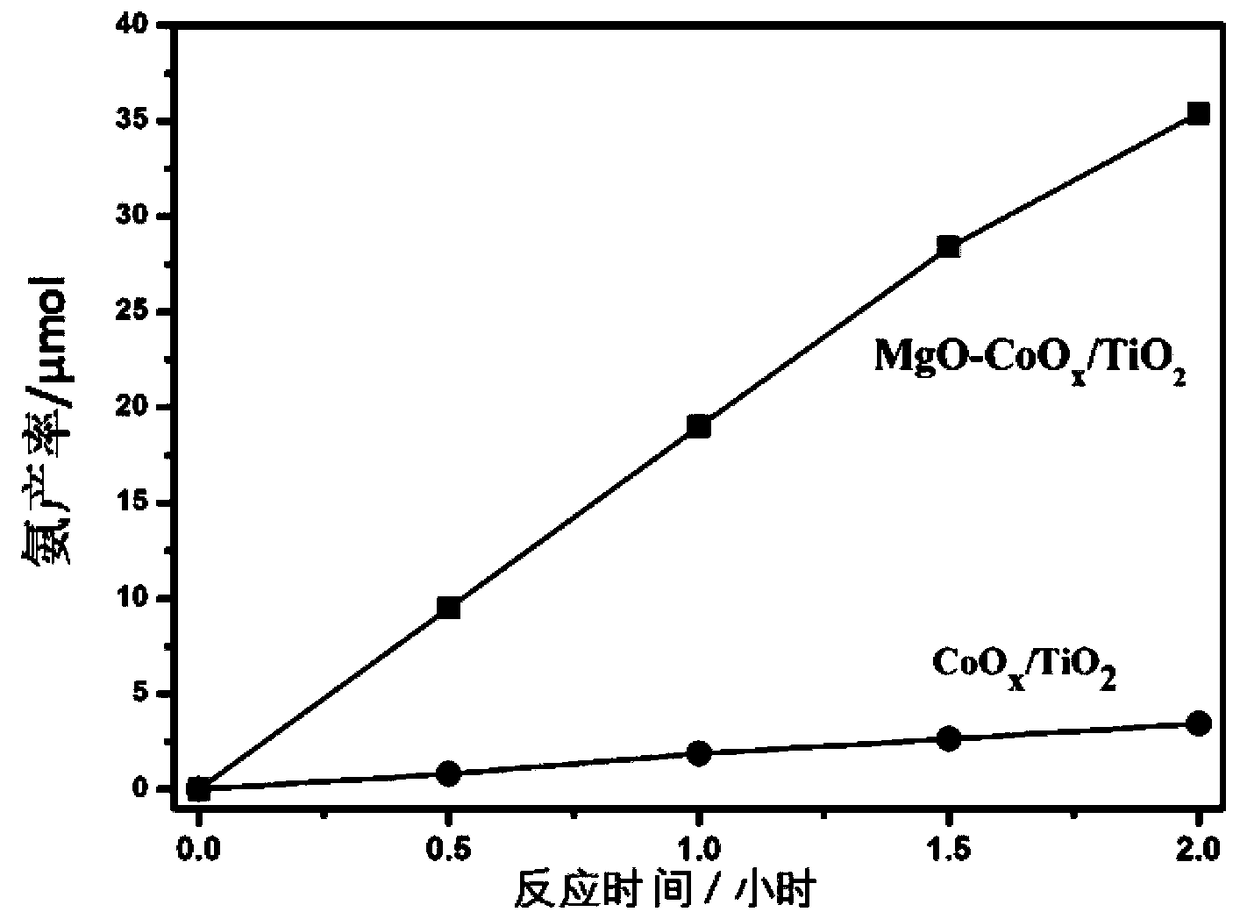
[0042] Experimental procedure comprises: 1. the MgO-CoO that makes in embodiment 1 x / TiO 2 The photocatalyst is put into the reactor, and the composite photocatalyst is dispersed in the reactor. 2. Feed N into the reactor 2 / H 2 O mixed gas. 3. Use a 500W xenon lamp to irradiate the photocatalyst in the reactor to make it react with the incoming gas. 4. Use 0.2mmol sulfuric acid aqueous solution as NH 3 The absorption solution, according to Nessler's reagent spectrophotometry for NH 3 Concentration detection. Figure 5 MgO-CoO shown x / TiO 2 Synthetic ammonia production-time graph, from Figure 5 It can be seen that the ammonia synthesis efficiency of the sample remains stable within 2 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com