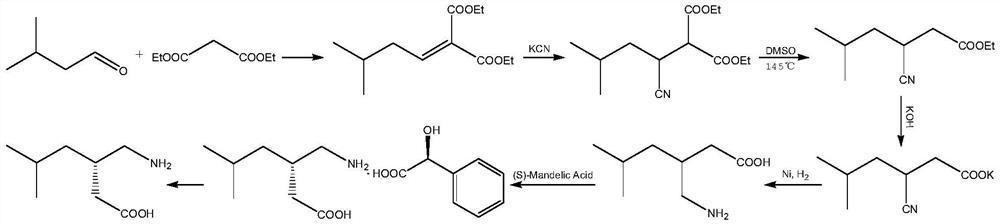
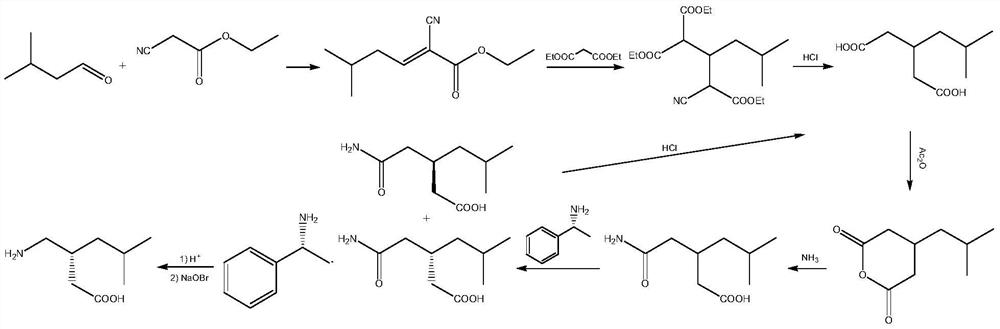
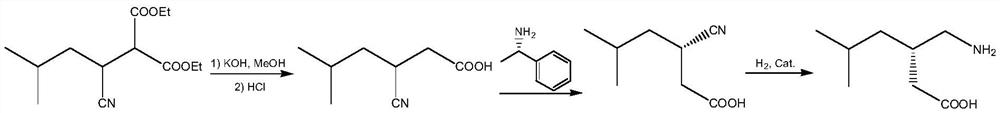
A green and efficient synthetic method of pregabalin
A pregabalin and synthesis method technology, applied in the direction of organic chemistry methods, chemical instruments and methods, hydrogen cyanide addition preparation, etc., can solve the problems of cumbersome pregabalin process route, increased production cost, poor atom economy, etc. Achieve the effects of shortening the reaction route, less environmental pollution, and reducing production energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1: the preparation of intermediate 1
[0073] Under the protection of argon, add 1.5L of ethyl acetate and 23.7g of sodium ethoxide into a dry three-necked flask, add 150g of isovaleraldehyde dropwise under stirring at room temperature, and the dropping time is 120min. Complete aldehyde conversion, stop stirring, adjust the pH value to 6-7 with 1N HCl aqueous solution under ice bath, separate the organic phase, extract the aqueous phase with ethyl acetate, combine the organic phase, wash the organic phase with saturated brine, and concentrate the organic phase , after rectification of the concentrated solution, 245.1 g of intermediate 1 (ethyl 5-methyl-2-hexenoate) was obtained, with a yield of 90.1%.
[0074] 1 H NMR (400MHz, DMSO) δ6.92-6.77(m, 1H), 5.84(dt, J=15.6, 1.5Hz, 1H), 4.11(q, J=7.1Hz, 2H), 2.11-2.07(m, 2H), 1.79-1.69(m, 1H), 1.21(t, J=7.1Hz, 3H), 0.88(d, J=6.7Hz, 6H).
Embodiment 2
[0075] Embodiment 2: the preparation of intermediate 1
[0076] Under the protection of argon, add 1.5L of ethyl acetate and 23.7g of sodium ethoxide into a dry three-necked flask, add 150g of isovaleraldehyde dropwise under stirring at room temperature, and the dropping time is 30min. Complete aldehyde conversion, stop stirring, adjust the pH value to 6-7 with 1N HCl aqueous solution under ice bath, separate the organic phase, extract the aqueous phase with ethyl acetate, combine the organic phase, wash the organic phase with saturated brine, and concentrate the organic phase , obtain 224g intermediate 1 (5-methyl-2-hexenoic acid ethyl ester) after rectification of concentrated solution, yield 82.5%, 1 H NMR is the same as in Example 1.
Embodiment 3
[0077] Embodiment 3: the preparation of intermediate 1
[0078]Under the protection of argon, add 1.5L of ethyl acetate and 23.7g of sodium ethoxide into a dry three-necked flask, add 150g of isovaleraldehyde dropwise under stirring at room temperature, and the dropping time is 180min. Complete aldehyde conversion, stop stirring, adjust the pH value to 6-7 with 1N HCl aqueous solution under ice bath, separate the organic phase, extract the aqueous phase with ethyl acetate, combine the organic phase, wash the organic phase with saturated brine, and concentrate the organic phase , obtain 243.5g intermediate 1 (5-methyl-2-hexenoic acid ethyl ester) after rectification of concentrated solution, yield 89.5%, 1 H NMR is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com