Synthesis method of lobeline
A synthesis method and compound technology, applied in the synthesis field of lobeline, can solve the problems of low yield, harsh reaction conditions, long route and the like, and achieve the effects of high yield, mild reaction conditions and concise process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
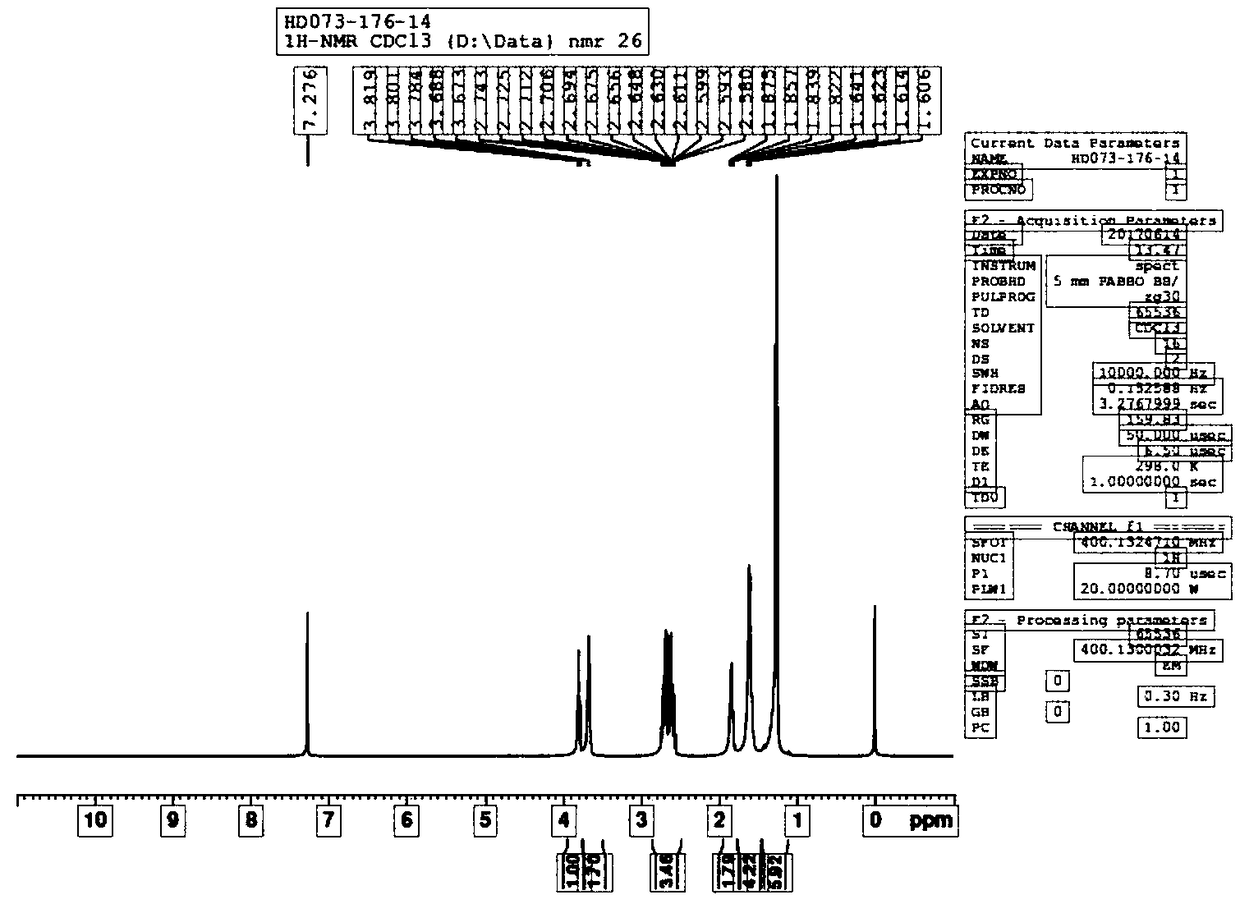
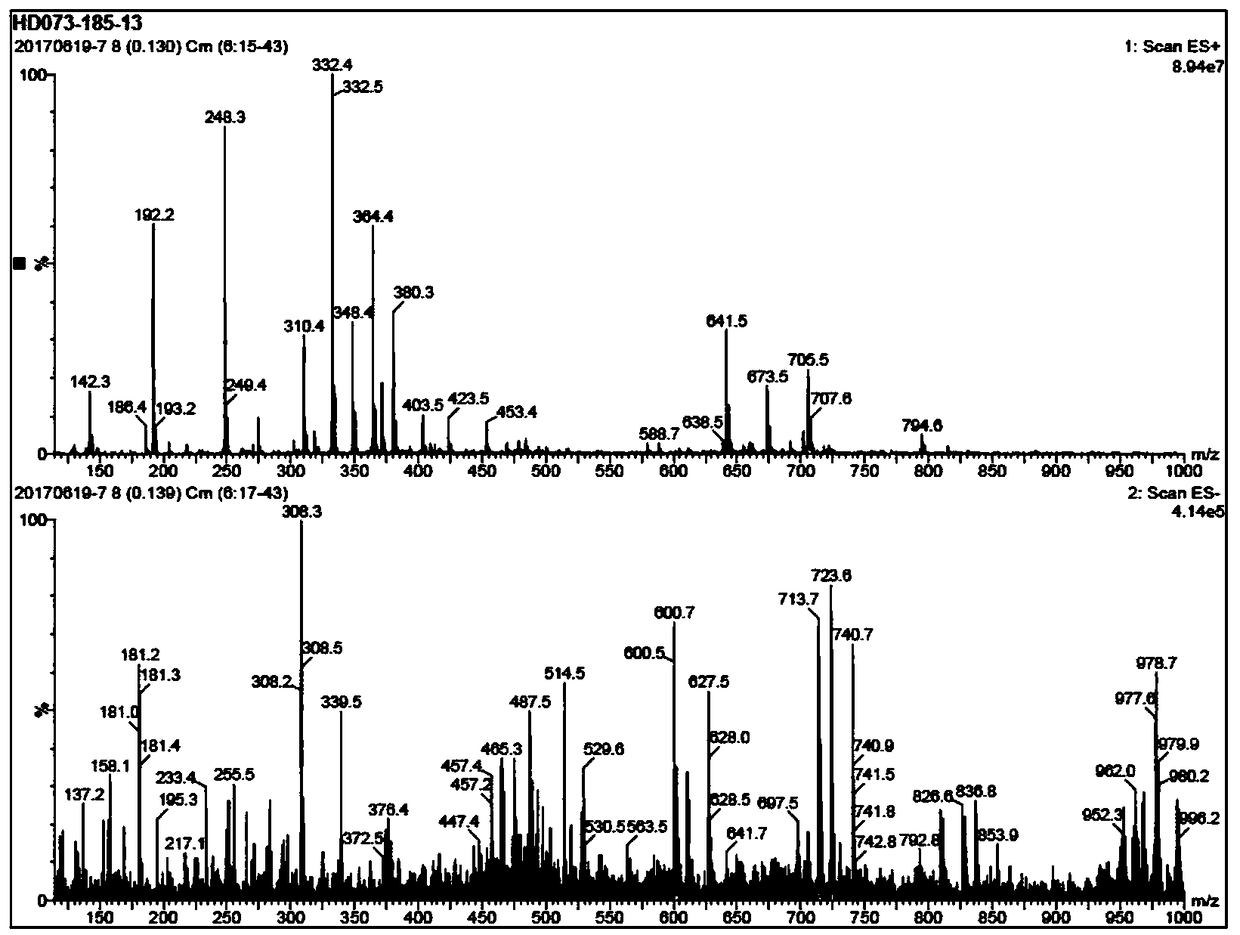
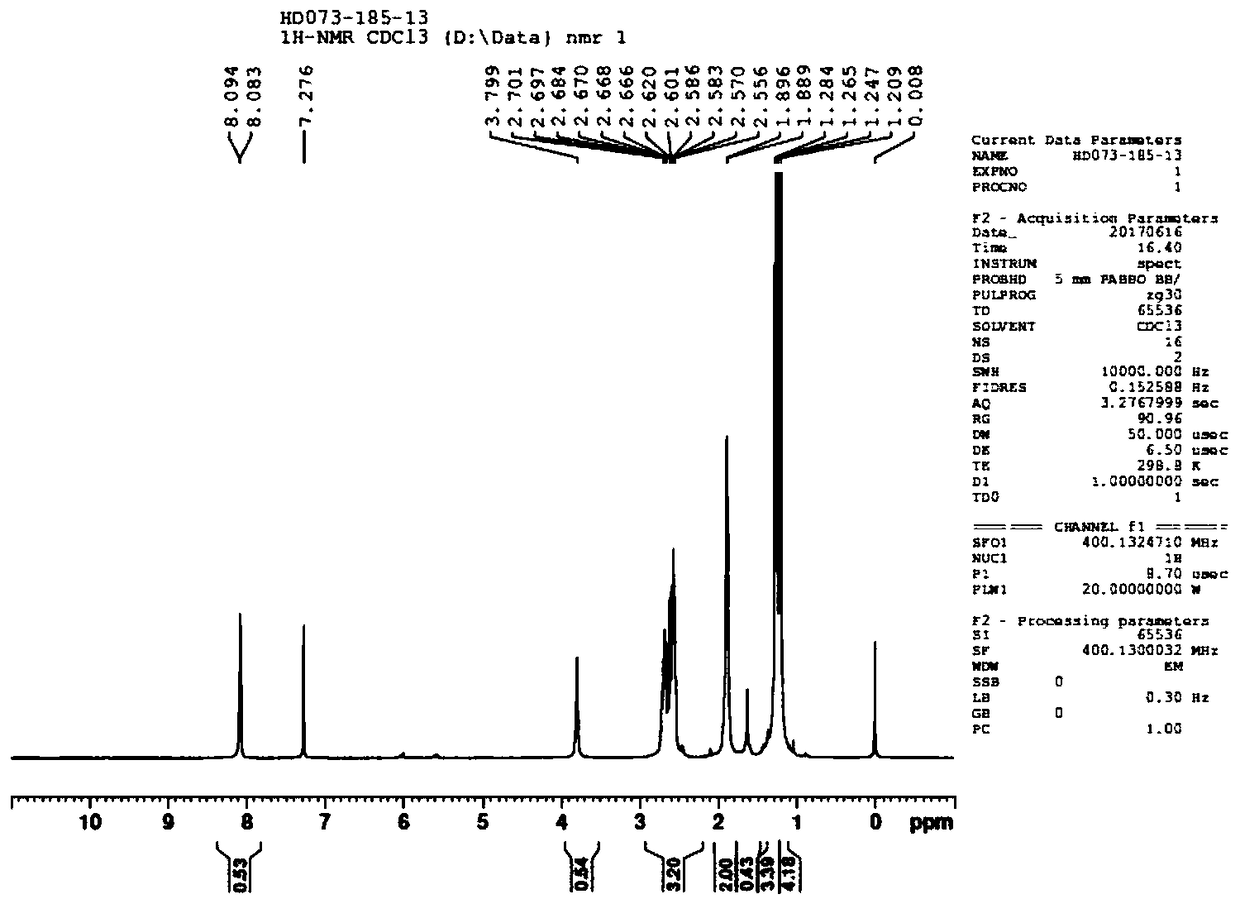
Image
Examples
preparation example Construction
[0053] This synthetic method comprises the steps:
[0054] (1) Ring-opening reaction: compound 1 is added dropwise to Lewis acid in ethanethiol medium to react to obtain compound 2;
[0055] (2) Oxidation reaction: Compound 2 is added dropwise to phosphoric acid in the medium of dicyclohexylcarbodiimide to obtain compound 3;
[0056] (3) Dehydration reaction: compound 3 is added to the medium of S-tert-butylsulfinamide, and water-absorbing agent anhydrous copper sulfate is added to react to obtain compound 4;
[0057] (4) Addition reaction: compound 4 reacts to obtain compound 5 under the action of acetophenone base;
[0058] (5) Reduction reaction: Compound 5 is stirred and reacted in a reducing agent medium, acid is added dropwise, the pH is adjusted to 2-3, and compound 6 is obtained by reaction;
[0059] (6) Protection reaction: compound 6 is reacted to obtain compound 7 in imidazole and TBSCl in a medium;
[0060] (7) Substitution reaction: Compound 7 was added dropwis...
Embodiment 1
[0065] 1. Pour 3.40kg (40.4mol, 1.0eq) of 3,4-dihydropyran into the cleaned 30-liter reactor, and then pour 6kg of toluene;
[0066] Under the protection of nitrogen, the temperature of ice brine is cooled. When the temperature drops below 10°C, 6.28kg of ethanethiol (101mmol, 2.5eq) is injected, and then 0.8kg of toluene is injected, and the temperature of ice brine is cooled under the protection of nitrogen;
[0067] When the temperature drops below 0°C, slowly add 0.20 kg of boron trifluoride diethyl ether dropwise within 1-2 hours;
[0068] After dropping 0.20kg, slowly drop the remaining 2.90kg of boron trifluoride ether into the reactor, and the temperature during the dropping process should not exceed 5°C;
[0069] After dripping 2.90kg of boron trifluoride diethyl ether, take a sample and submit it for inspection. After confirming that the reaction of the raw materials is complete (3,4-dihydropyran < 1%), the reaction solution is quenched;
[0070] For the configurati...
Embodiment 2
[0122] 1. Pour 34kg of 3,4-dihydropyran into the cleaned 300-liter reactor, and then pour 60kg of toluene into it, and cool it down with ice salt water under the protection of nitrogen. When the temperature drops below 10°C, pour 64kg of ethanethiol (2.5 eq), then inject 8kg of toluene, and cool the ice salt water under the protection of nitrogen;
[0123] When the temperature drops below 0°C, start to slowly add 2kg of boron trifluoride diethyl ether dropwise within 1-2 hours;
[0124] After dropping 2kg, slowly drop the remaining boron trifluoride diethyl ether 29kg (0.5eq) into the reactor, and the temperature during the dropping process should not exceed 5°C;
[0125] After dripping 29kg of boron trifluoride ether, take a sample and submit it for inspection. After confirming that the reaction of the raw materials is complete (3,4-dihydropyran < 1%), the reaction solution is quenched;
[0126] For the configuration of saturated aqueous sodium bicarbonate solution, 9kg of s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com