Barbus tetrazona heat shock protein PtHsp70 and application thereof
A heat shock protein, tiger skin technology, applied in the field of genetic engineering, can solve the problems of sequence structure and expression mechanism that have not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 PtHSP70 gene cloning
[0018] Three 8-month-old tiger skin fishes cultured in our laboratory were taken, anesthetized with MS-222, dissected, and the brain, gills, liver and muscle tissues were collected respectively, and the same kinds of tissues were mixed and stored, and the total RNA was immediately extracted with TRizol reagent. The Nanodrop2000 Nucleic Acid Protein Concentration Detector was used to measure the total RNA concentration and contamination, the RNA integrity was detected by agarose gel electrophoresis, and stored at -80°C for later use.
[0019] Using the total RNA of each tissue as a template, the first strand of cDNA was synthesized using a reverse transcription kit (TaKaRa, 6110A) according to the operating method and reagent instructions. Chain polymerase reaction (PCR) was performed using the first strand of cDNA as a template, in which primer F1: 5'-TCGAAGCGAGCTCTGGTGATGGACGTGT-3', R1: 5'-TCACCAACGACAAGGGCAGGCTGAGCAA-3'. PCR reaction ...
Embodiment 2
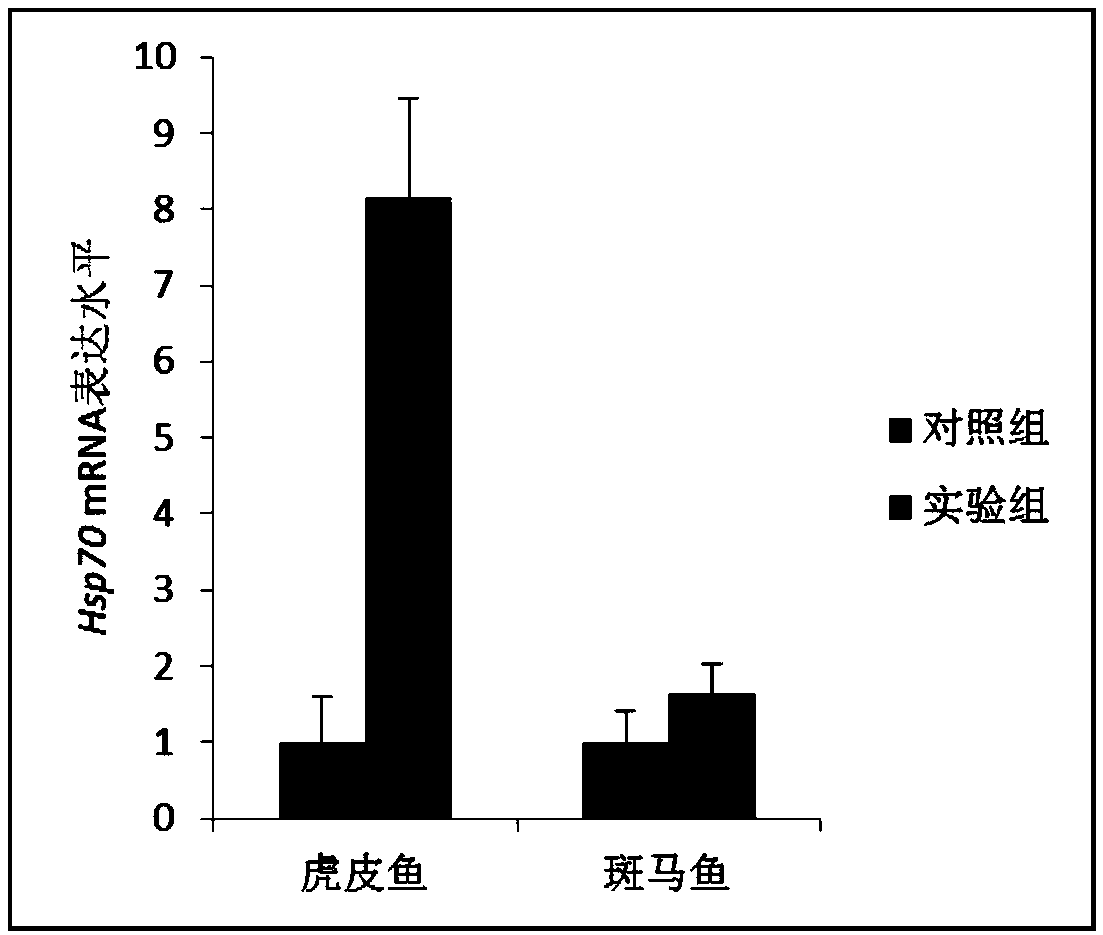
[0021] Example 2 Analysis of HSP70 gene expression level in tiger fish and zebrafish under low temperature stress
[0022] Tiger fish and zebrafish belong to the Cyprinidae family and are native to freshwater basins in Southeast Asia, and have a high degree of evolutionary similarity. Thirty 8-month-old adult tiger fish and zebrafish were randomly divided into experimental group and control group, with 15 fish in each group. Each group was divided into 3 tanks with 5 fish in each tank. Adopt the method of gradient cooling, cool down the tiger skin fish cultured at 27℃ by 2℃ every 24hr, when the temperature reaches 19℃, continue to culture at this temperature for 24hr, dissect muscle tissue after MS-222 anesthesia, and extract total RNA with TRizol reagent , using a reverse transcription kit (TaKaRa, 6110A) to synthesize the first strand of cDNA and use it as a template for qPCR experiments. Real-time fluorescent quantitative PCR (qPCR) technology was used to detect the mRNA e...
Embodiment 3
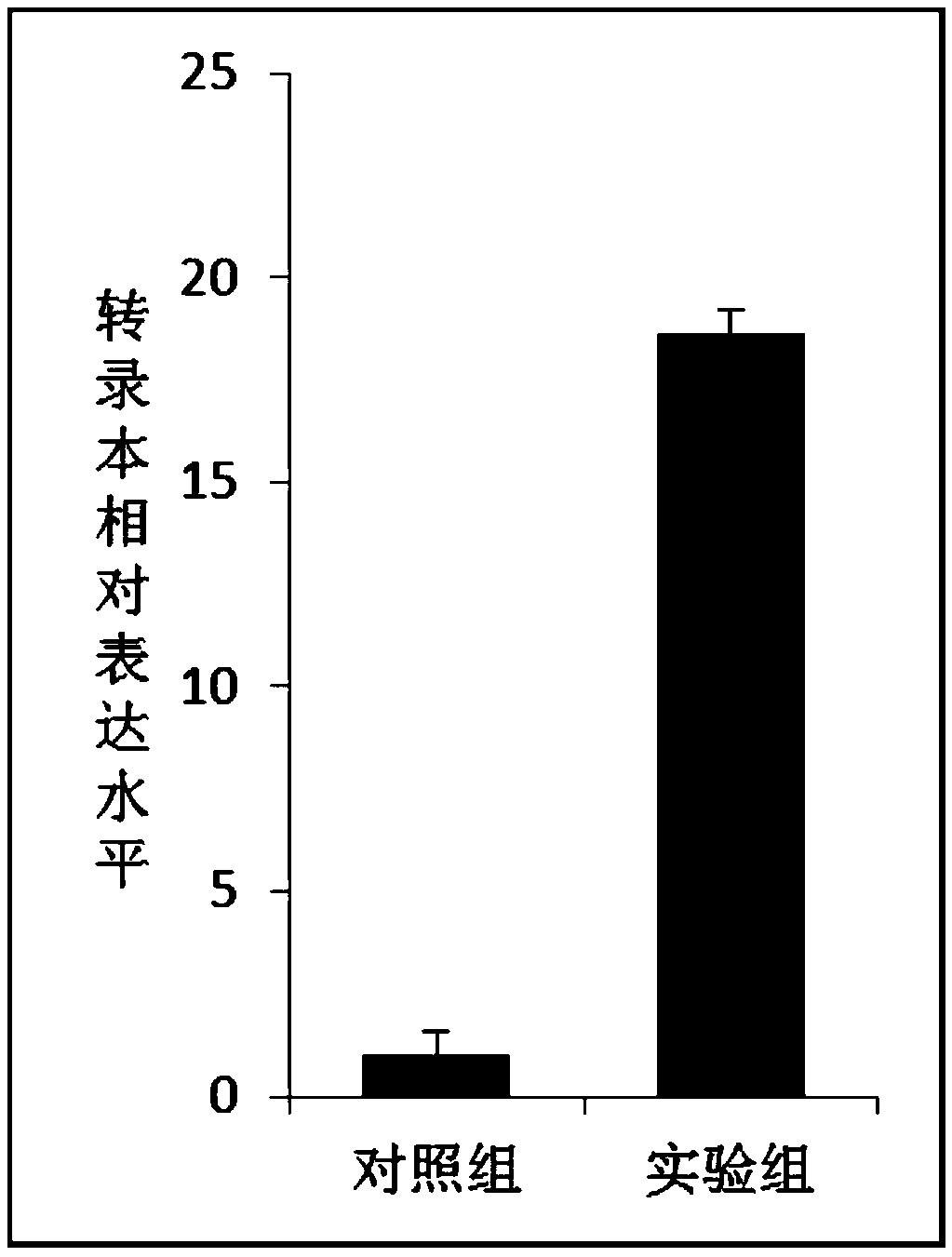
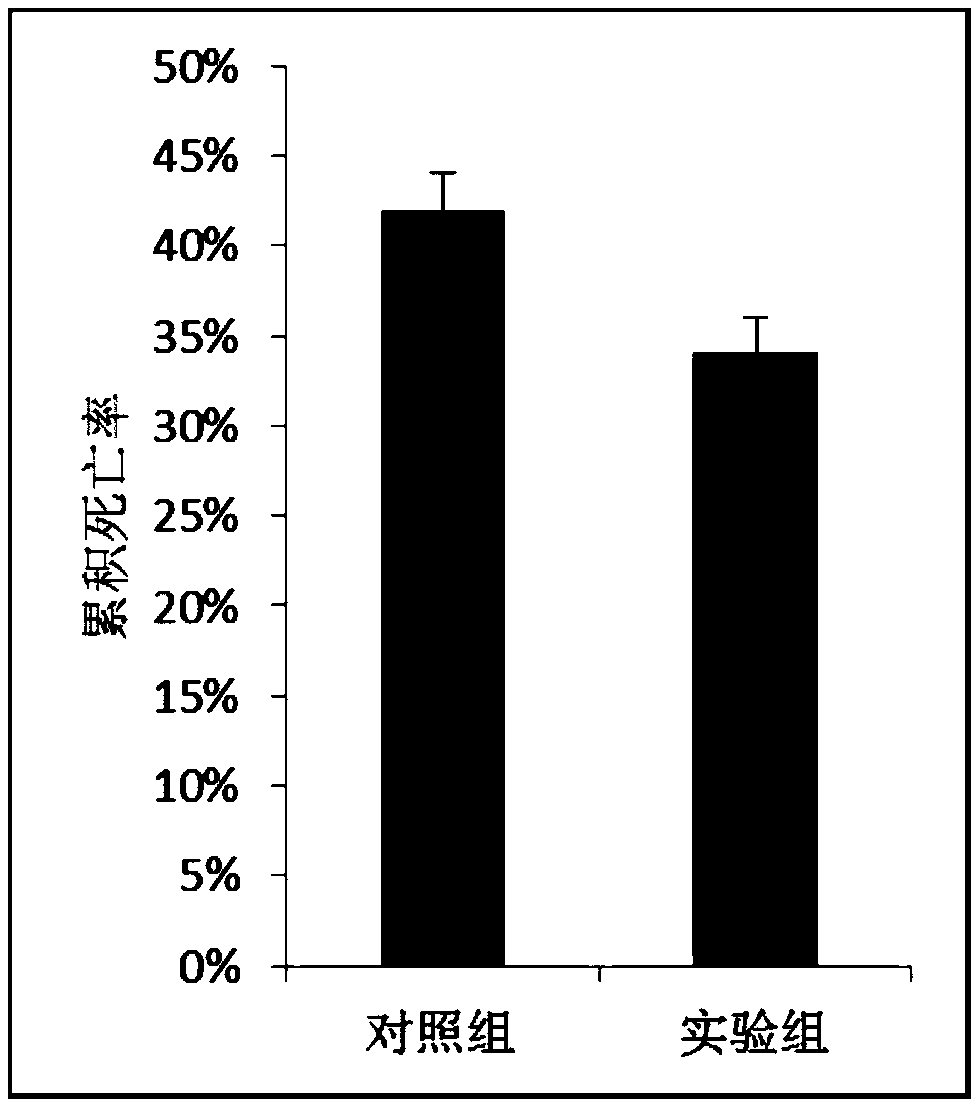
[0027] Example 3 Preparation of linearized vector and target fragment
[0028] Carry out Bgl II and Sal I double enzyme digestion on the commercialized plasmid pAcGFP1-C1. The enzyme digestion system is: 2 μL 10×Buffer O, 600 ng of pAcGFP1-C1 plasmid, 1 μL each of endonuclease Sal I and Bgl II, deionized water Bring the volume up to 20 μL.
[0029] Primers were designed according to the gene sequence information of the target fragment, and restriction sites agatct (Bgl II) and gtcgac (Sal I) were introduced into the upstream and downstream of the fragment respectively, wherein the sequence of primer F2 was: 5'-agatctATGTCATCTGCAAAAGGAGTTGCTATTG-3'; the sequence of primer R2 was : 5'-gtcgacTTAATCCACCTCCTCGATAGTGGGC-3', prepare the solution according to the following reaction system: 0.25μL Takara Ex Taq, 5μL 10×PCR buffer (Mg 2+ free), 4 μL MgCl 2 , 4μL dNTPmixture (0.25mM each), 0.4μL (<500ng) cDNA template, 2μL each of primer F (10μM) and primer R (10μM), make up the volume...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com