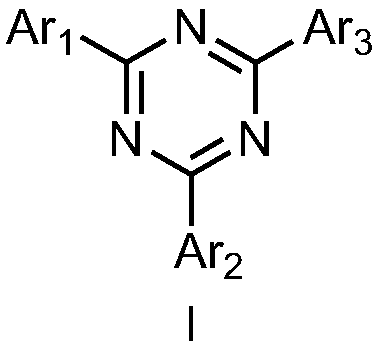
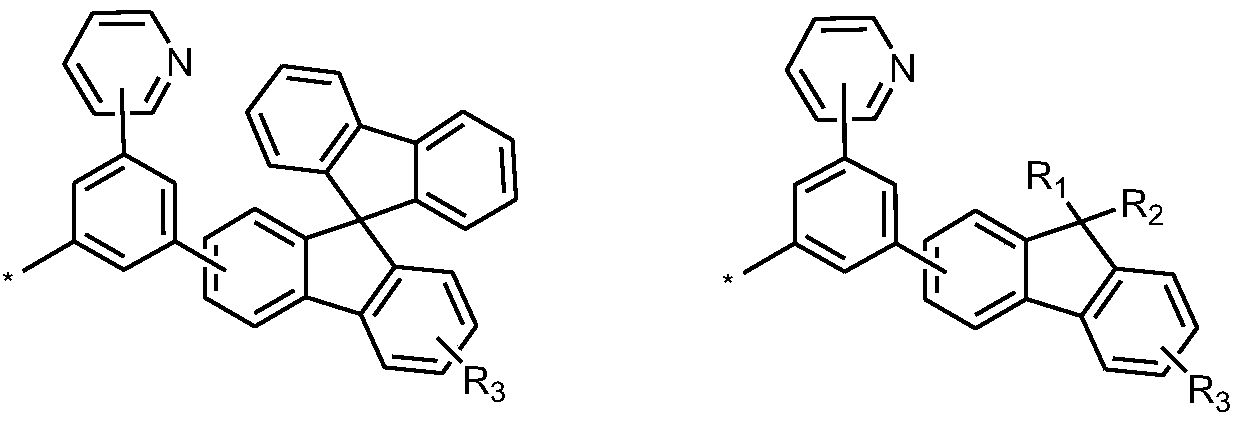
Triazine compound containing fluorine structure, and organic light-emitting devices thereof
A technology of organic light-emitting devices and triazine compounds, which is applied in the field of organic photoelectric materials, can solve problems such as device performance degradation, and achieve the effects of low driving voltage, high luminous efficiency, and cost savings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] [Example 1] Synthesis of Compound A1
[0058]
[0059] Synthesis of intermediate a1-1
[0060] Take 2-(3,5-dichlorophenyl)pyridine (9.5g, 42.4mmol) into a three-necked flask, add THF 100mL, protect with nitrogen, stir at -78°C for 30 minutes, then add n-butyllithium (2.5M) 21 mL, reacted for 1 hour, then added 14 g of triisopropyl borate, reacted at low temperature for 1 hour, and gradually returned to room temperature. In the post-treatment process, 2M hydrochloric acid was added to the system to make the pH of the solution 4-5, and the liquid separation was allowed to stand. The aqueous layer was extracted with ethyl acetate, the organic layers were combined, and spin-dried to obtain intermediate a1-1 (8.2g, yield 80%).
[0061] Mass Spectrum m / z: 242.86 (calculated: 242.83). Theoretical element content (%)C 11 h 11 B 2 NO 4 : C, 54.41; H, 4.57; B, 8.90; N, 5.77; O, 26.35 The measured element content (%): C, 54.41; The above results confirmed that the obtai...
Embodiment 2
[0068] [Example 2] Synthesis of Compound A13
[0069]
[0070] The 2-bromo-9,9-dimethylfluorene in Example 1 was replaced by equimolar 2-bromo-9,9-spirobifluorene, and the other steps were the same as the synthesis of Example 1 to obtain the target product compound A13. Mass Spectrum m / z: 1092.26 (calculated: 1092.29). Theoretical element content (%)C 81 h 49 N 5 : C, 89.07; H, 4.52; N, 6.41 Measured element content (%): C, 89.08; H, 4.51; N, 6.41. The above results confirmed that the obtained product was the target product.
Embodiment 3
[0071] [Example 3] Synthesis of Compound A27
[0072]
[0073] In Example 1, the 2-bromo-9,9-dimethylfluorene was replaced by equimolar 2-bromo-9,9-diphenylfluorene, and 2-(3,5-dichlorobenzene)pyridine was replaced by Equimolar 3-(3,5-dichlorophenyl)pyridine, and other steps were the same as in Example 1 to obtain the target compound A27. Mass Spectrum m / z: 1096.26 (calculated: 1096.32). Theoretical element content (%)C 81 h 53 N 5: C, 88.74; H, 4.87; N, 6.39 Measured element content (%): C, 88.76; H, 4.86; N, 6.38. The above results confirmed that the obtained product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com