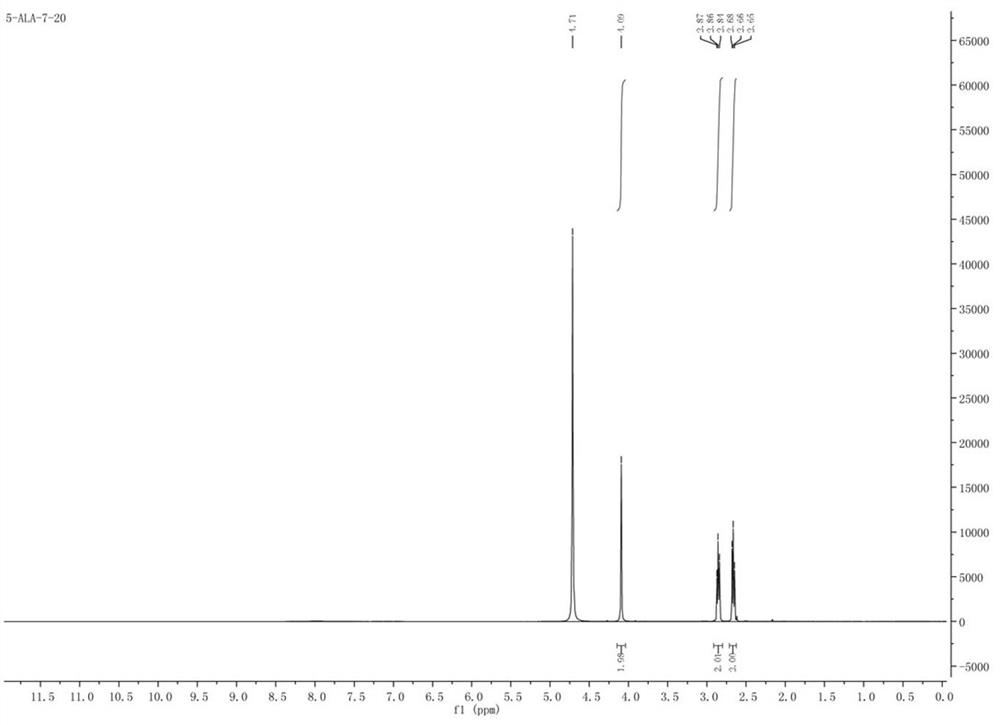
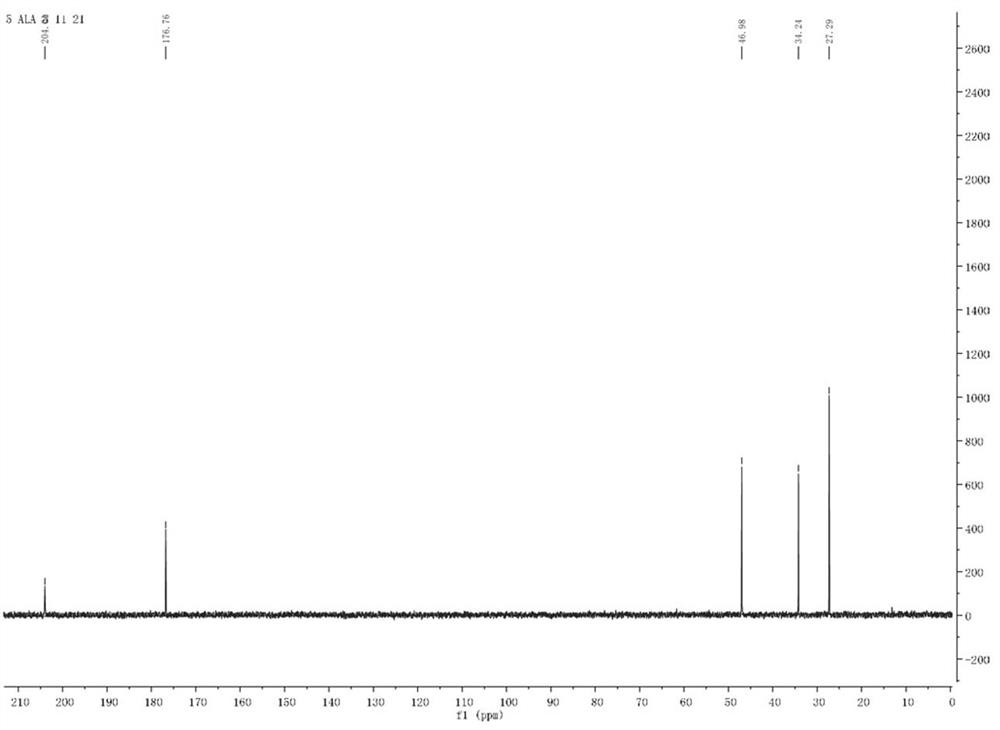
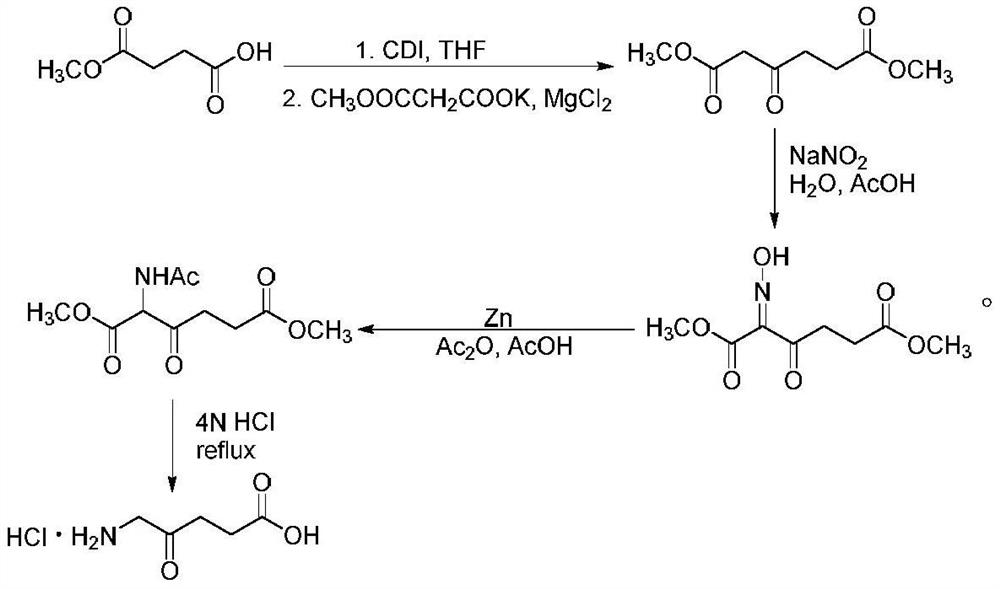
A kind of synthetic method of 5-aminolevulinic acid hydrochloride
A technology of aminolevulinic acid hydrochloride and synthesis method, applied in chemical instruments and methods, preparation of carboxylate, preparation of organic compounds, etc., can solve the problem that ethyl bromoacetoacetate has large environmental pollution and is not suitable for mass industrialization The problems of low production and reaction yield, etc., achieve the effects of low synthesis cost, less three wastes, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The synthesis of 5-aminolevulinic acid hydrochloride comprises the following steps:
[0043] Step 1: Synthesis of Monomethyl Succinate
[0044] Dry methanol (112.14 g, 3500 mmol) was added to a 500 mL single-necked flask, and succinic anhydride (100.07 g, 1000 mmol) was added to the single-necked flask under magnetic stirring. After the raw materials were added, the temperature was raised to 80°C, heated to reflux, and reacted for 3 hours. After the reaction, the reaction solution was in a clear state, and the reaction solution was subjected to vacuum distillation (using a water pump to carry out vacuum distillation), steamed methanol, and the remaining liquid was cooled to room temperature, crystallized overnight, and suction filtered the next day to obtain a white solid, namely Monomethyl succinate, yield 97%.
[0045] Step 2: Synthesis of methyl 4-(1-imidazole)-4-oxobutanoate
[0046] Add dry dichloromethane (400 mL) into a 2000 mL three-necked flask, stir at 0°C,...
Embodiment 2
[0052] The synthesis of 5-aminolevulinic acid hydrochloride comprises the following steps:
[0053] Step 1: Synthesis of Monomethyl Succinate
[0054] Dry methanol (128.17g, 4000mmol) was added to a 500mL single-necked flask, and succinic anhydride (100.07g, 1000mmol) was added to the single-necked flask under magnetic stirring. After the raw materials were added, the temperature was raised to 75°C, heated to reflux, and reacted for 5 hours. After the reaction, the reaction solution was in a clear state. The reaction solution was distilled under reduced pressure (using a water pump to carry out vacuum distillation), methanol was distilled off, the remaining liquid was cooled to room temperature, crystallized overnight, and a white solid was obtained by suction filtration the next day, which was Monomethyl succinate, yield 96%.
[0055] Step 2: Synthesis of methyl 4-(1-imidazole)-4-oxobutanoate
[0056] Add dry tetrahydrofuran (400 mL) into a 2000 mL three-necked flask, stir...
Embodiment 3
[0062] The synthesis of 5-aminolevulinic acid hydrochloride comprises the following steps:
[0063] Step 1: Synthesis of Monomethyl Succinate
[0064] Dry methanol (144.19 g, 4500 mmol) was added to a 500 mL single-necked flask, and succinic anhydride (100.07 g, 1000 mmol) was added to the single-necked flask under magnetic stirring. After the raw materials were added in, the temperature rose to 78°C, heated to reflux, and reacted for 4 hours. After the reaction, the reaction solution was in a clear state. The reaction solution was distilled under reduced pressure (using a water pump to carry out vacuum distillation), methanol was distilled off, the remaining liquid was cooled to room temperature, crystallized overnight, and a white solid was obtained by suction filtration the next day, which was Monomethyl succinate, yield 95%.
[0065] Step 2: Synthesis of methyl 4-(1-imidazole)-4-oxobutanoate
[0066] Add dry dichloroethane (400 mL) into a 2000 mL three-necked flask, sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com