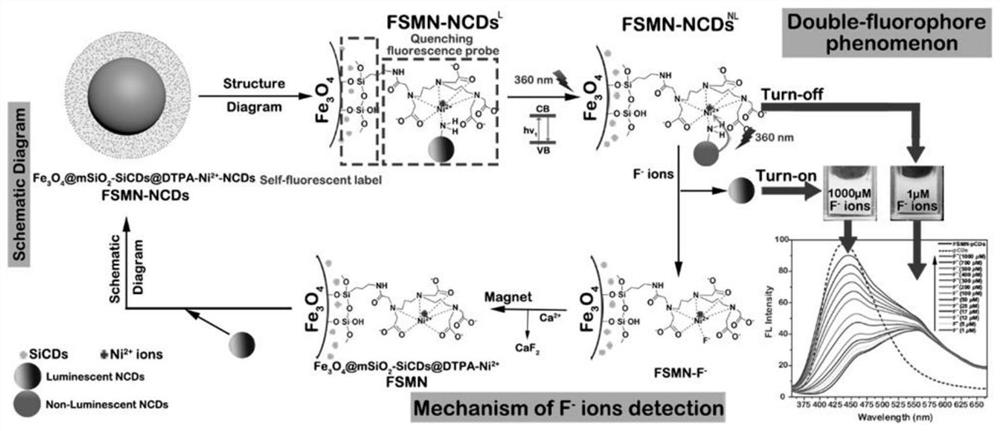
A method for detecting and removing fluoride ions
A detection method, fluorine ion technology, applied in chemical instruments and methods, measuring devices, nanotechnology for materials and surface science, etc., can solve the problem of low selectivity and sensitivity of metal ions, and unsatisfactory dispersion , probe biological toxicity and other issues, to achieve excellent superparamagnetic properties, good cell permeability, and low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] Embodiment 1 hydrophilic Fe 3 o 4 Synthesis of cores, organosilane-functionalized carbon dots (SiCDs) in dilute solutions and autofluorescent markers (Fe 3 o 4 @mSiO 2 -SiCDs) preparation
[0119] 1. Hydrophobic Fe 3 o 4 The synthesis of the core includes the following steps:
[0120] (1) 0.325g anhydrous FeCl 3 and 0.4g of trisodium citrate were dissolved in 40mL of ethylene glycol, and magnetically stirred until an orange-yellow solution was formed;
[0121] (2) Under magnetic stirring, add 3 g of sodium acetate until a uniform yellow-brown solution is obtained, transfer the solution to a 50 mL Teflon-lined autoclave, and keep it at 200 ° C for 10 h; cool to room temperature Finally, the black product was separated by magnetic force, washed 5 times with ethanol, washed 1 time in deionized water, and dried in vacuum at 40 °C to obtain the hydrophobic Fe 3 o 4 core.
[0122] 2. The synthesis of organosilane functionalized carbon dots (SiCDs) dilute solution, ...
Embodiment 2
[0131] Embodiment 2DTPA acid anhydride and the magnetic nanoparticle (Fe 3 o 4 @mSiO 2 -SiCDs@DTPA-Ni 2+ , FSMN) preparation
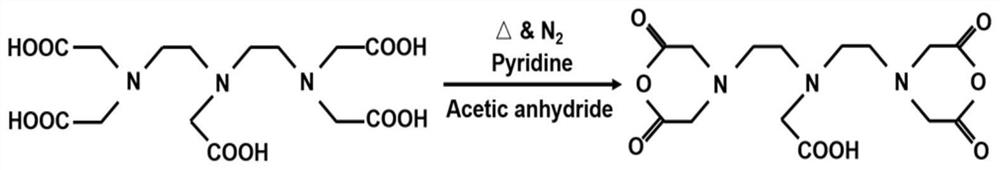
[0132] 1, the preparation process of diethylenetriaminepentaacetic acid (DTPA) anhydride is as follows figure 1 As shown, it specifically includes the following steps:
[0133] (1) 33.7g of diethylenetriaminepentaacetic acid (DTPA) was added to a 250mL round bottom flask containing 40mL of pyridine, and then 33mL of acetic anhydride was added;
[0134] (2) Pass N 2 Dissolved oxygen was removed, and the solution was vigorously stirred (1000 rpm) at 70°C for 24 hours to obtain DTPA anhydride;
[0135] (3) Wash DTPA anhydride twice in excess acetic anhydride, then wash three times in excess ether, and dry in vacuum to obtain diethylenetriaminepentaacetic dianhydride.
[0136] 2. Fluorescent silica-coated magnetic nanoparticles (Fe 3 o 4 @mSiO 2 -SiCDs@DTPA-Ni 2+ , FSMN) preparation method, comprises the following steps:
[0137] (1) the Fe of ...
Embodiment 3
[0140] Synthesis of embodiment 3 pure carbon dots (NCDs) and fluorescent probe (Fe 3 o 4 @mSiO 2 -SiCDs@DTPA-Ni 2+ -NCDs, FSMN-NCDs) preparation
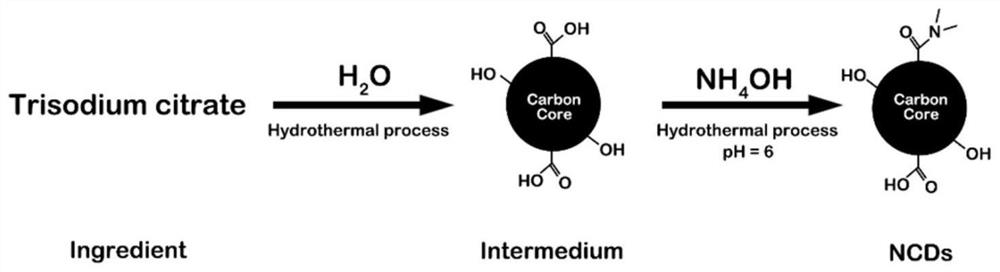
[0141] 1. The preparation process of pure carbon dots (NCDs) is as follows: figure 2 As shown, it specifically includes the following steps:
[0142] (1) Mix 20 mL of deionized water with 0.84 g of trisodium citrate, transfer it to a 50 mL polytetrafluoroethylene-lined hydrothermal reactor, and react at a constant temperature of 200 ° C for 5 h;
[0143] (2) After cooling, add ammonia solution and react at 200°C for another 10 hours, dialyze the obtained yellow-brown solution in a dialysis bag, soak it in 1000mL deionized water, and stir it with gentle magnetic force (200~600rpm) at the same time overnight;
[0144] (3) After adjusting the pH value to 6, the NCDs solution was obtained.
[0145] 2. Fluorescent probes (Fe 3 o 4 @mSiO 2 -SiCDs@DTPA-Ni 2+ -NCDs, the preparation method of FSMN-NCDs) is as follows:
[0146] U...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com