Method for quantitatively detecting content of intermediate in production process of recombinant human interferon alpha by affinity chromatography,
A technology for quantitative detection of interferon-alpha, applied in the field of biomedicine, can solve problems such as the difficulty in obtaining a large amount of ligand antibodies, and achieve the effects of easy evaluation, improved cognition and control, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1 Obtaining and affinity analysis of anti-recombinant human interferon alpha monoclonal antibody
[0064] The immune antigen used in the present invention is purified recombinant human interferon alpha, and the immune animals are 6-week-old male BALB / C mice. 40 monoclonal antibody-positive cell lines against recombinant human interferon-alpha were obtained. Among them, BTP-mAb4D9D1E7 is a monoclonal antibody cell line secreting anti-recombinant human alpha interferon and recombinant integrated interferon. The titer of BTP-mAb4D9D1E7 ascites monoclonal antibody is 1:10000-30000. Continuously passaged in vitro for 6 months, the ability to secrete antibodies is stable and specific; the subclass of monoclonal antibodies is IgG 1 .
[0065] The specificity and affinity analysis of the monoclonal antibody to recombinant human alpha interferon and recombinant integrated interferon is as follows:
[0066] Coat 1-10 μg / ml recombinant human alpha interferon and recomb...
Embodiment 2
[0069] Example 2 Method for Quantitative Detection of Recombinant Human Alpha Interferon Alpha 1b Production Process Intermediate Content by Affinity Chromatography
[0070] 1. Construction of engineering bacteria expressing recombinant human alpha interferon alpha 1b
[0071] The gene encoding the recombinant human interferon alpha 1b is inserted into the expression vector pET-23b to obtain a recombinant plasmid, and then the recombinant plasmid is used to transform Escherichia coli BL21 to obtain a recombinant engineered bacterium. The recombinant engineering bacteria were fermented and cultured to induce expression. For details, please refer to CN201010189352.8.
[0072] 2. Ferment engineering bacteria and isolate and purify the target protein expressed in the form of soluble protein or inclusion body
[0073] Protein purification includes crude purification and purification. Ammonium sulfate salt precipitation is used to obtain the crude protein product. The crude produc...
Embodiment 3
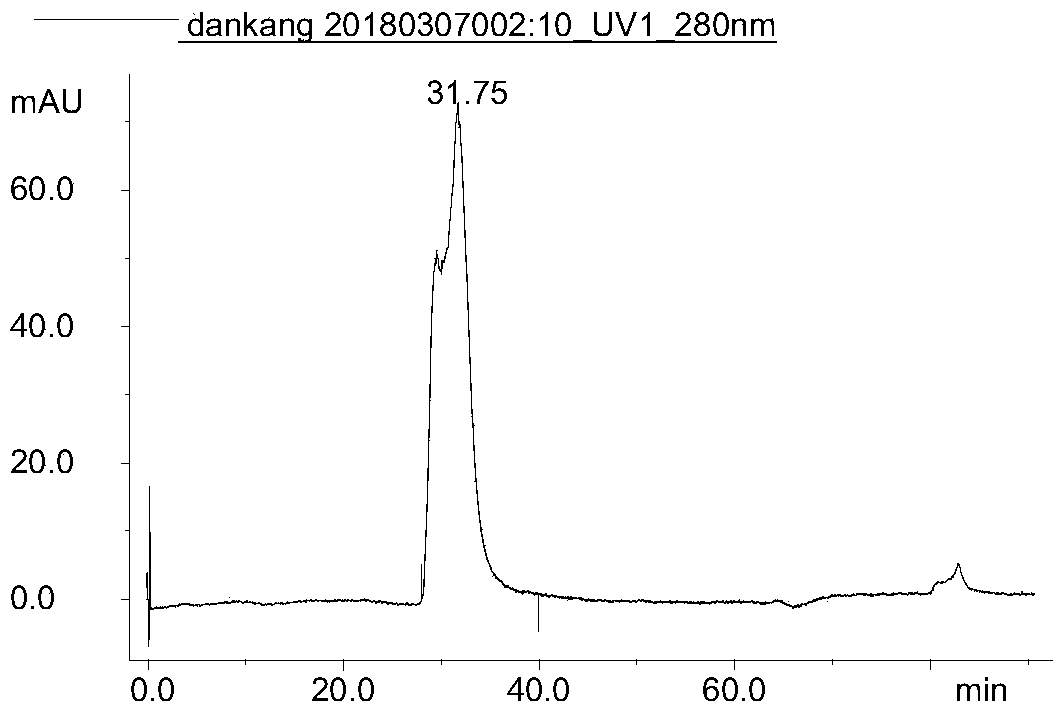
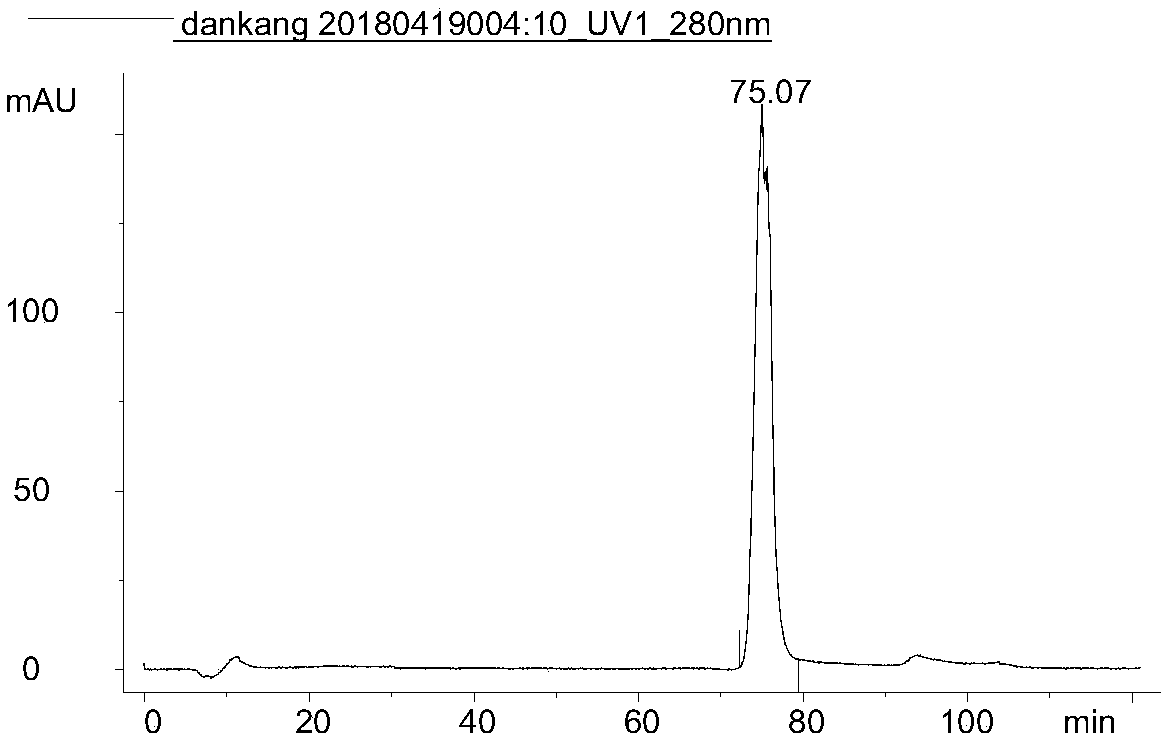
[0087] Example 3 Immunoaffinity Chromatography Quantitative Detection of Interferon Process Intermediate Accuracy Verification
[0088] Experimental materials: Instrument: AKTA purifier, chromatographic column: the monoclonal antibody affinity chromatography column of Example 1, mobile phase A: 25mM PBS, pH 7.0, mobile phase B: 0.1M Gly-0.2M NaCl, adjust the pH to 2.5. Loading sample: recombinant human interferon α1b standard substance, the concentration is 5 mg / ml.
[0089] Experimental scheme: flow rate: 1.274cm / min, detection wavelength: 280nm, detection time: 65min, elution condition: 100%A(3CV)-100%B(6CV)-100%A(5CV).
[0090] Experimental procedure: After equilibrating the column with mobile phase A, load 0.2ml, 0.3ml, 0.4ml, and 1ml of interferon standard in sequence, completely collect the elution peak of the target protein, adjust the pH to neutral with NaOH, and detect with the trace lowry method concentration.
[0091] Experimental results: the recovery rate was ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com