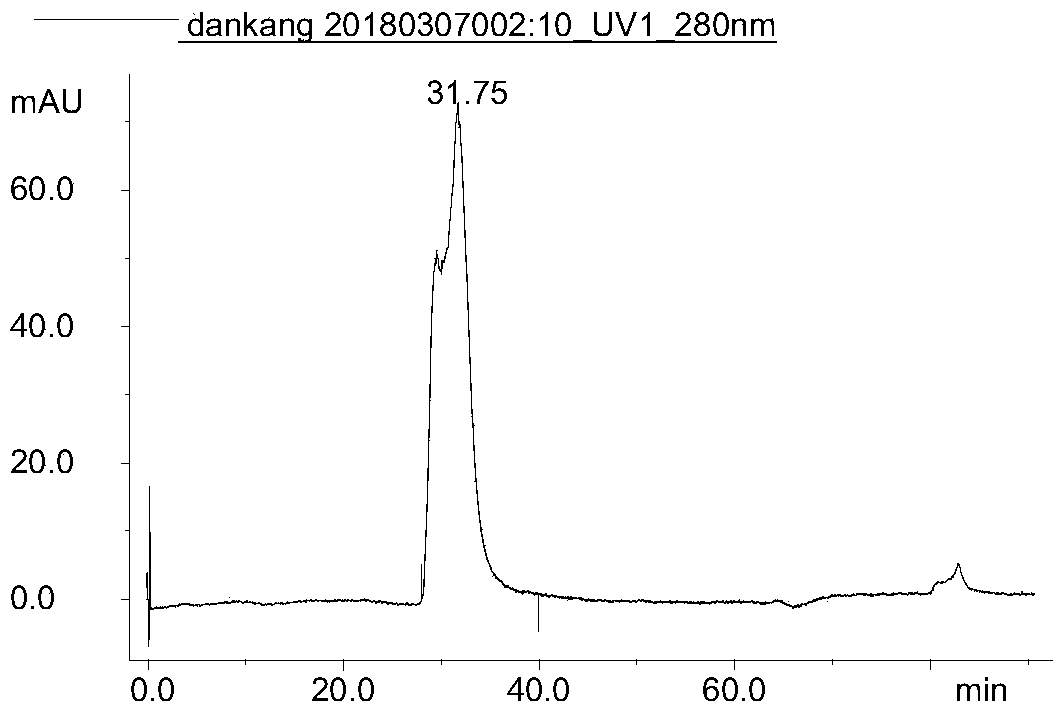
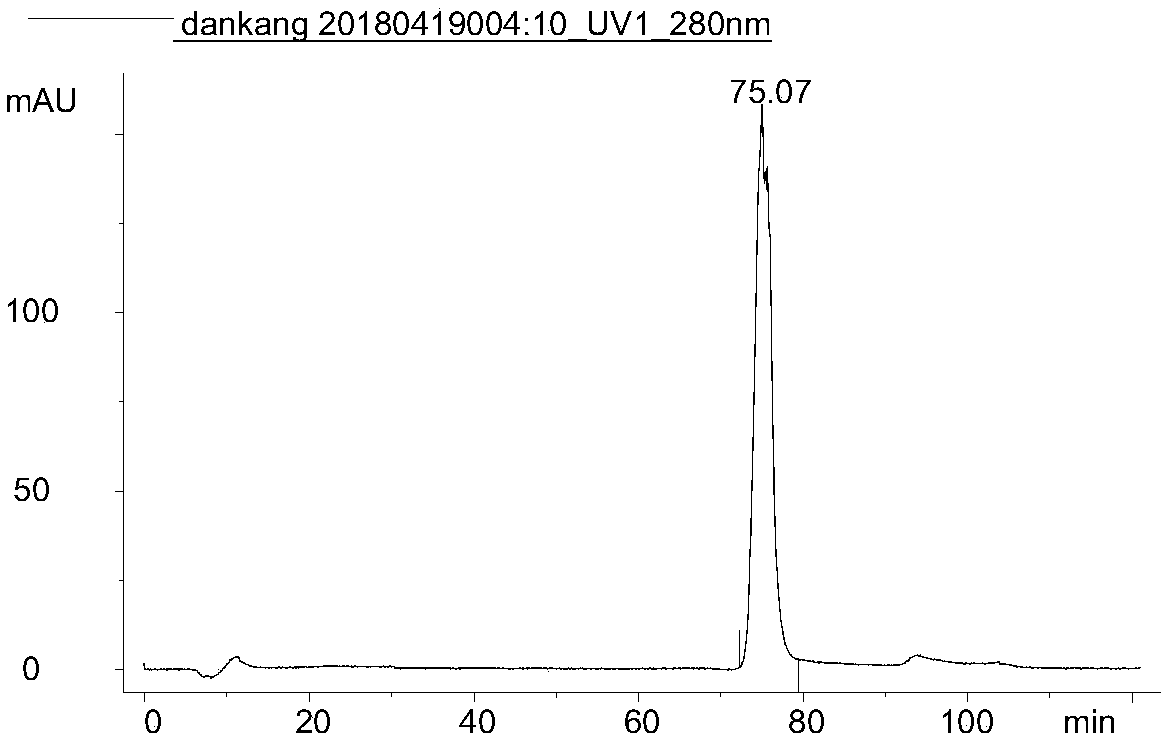
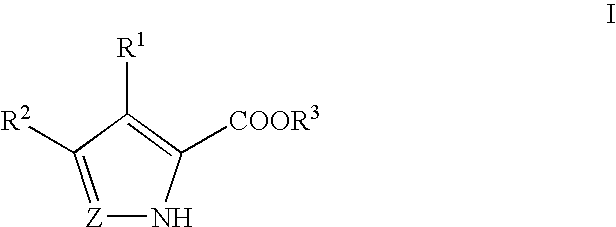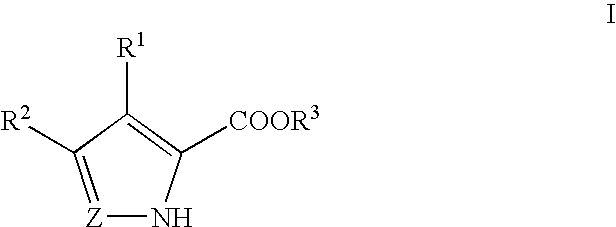Patents
Literature
37results about How to "Improve cognition" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
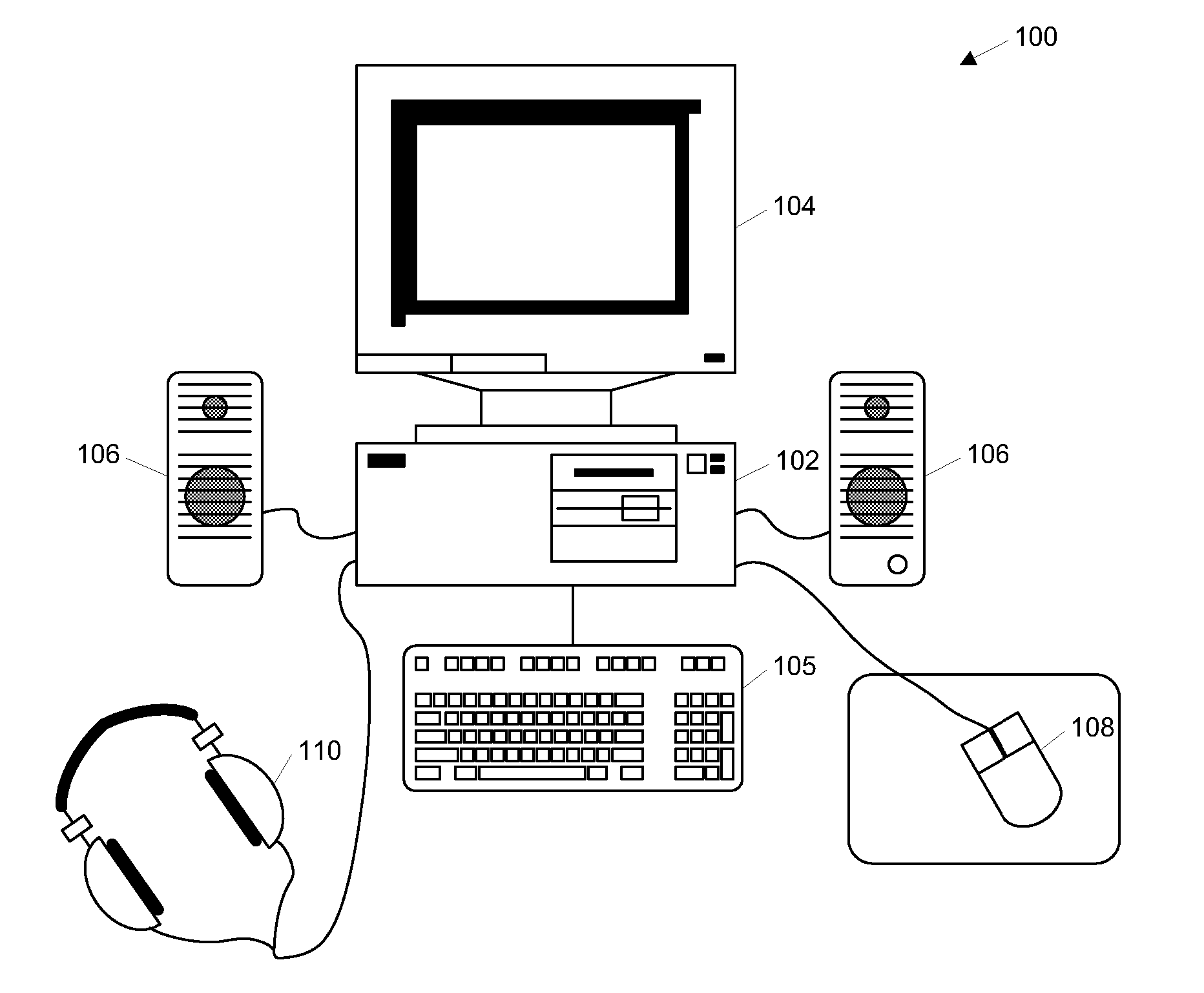
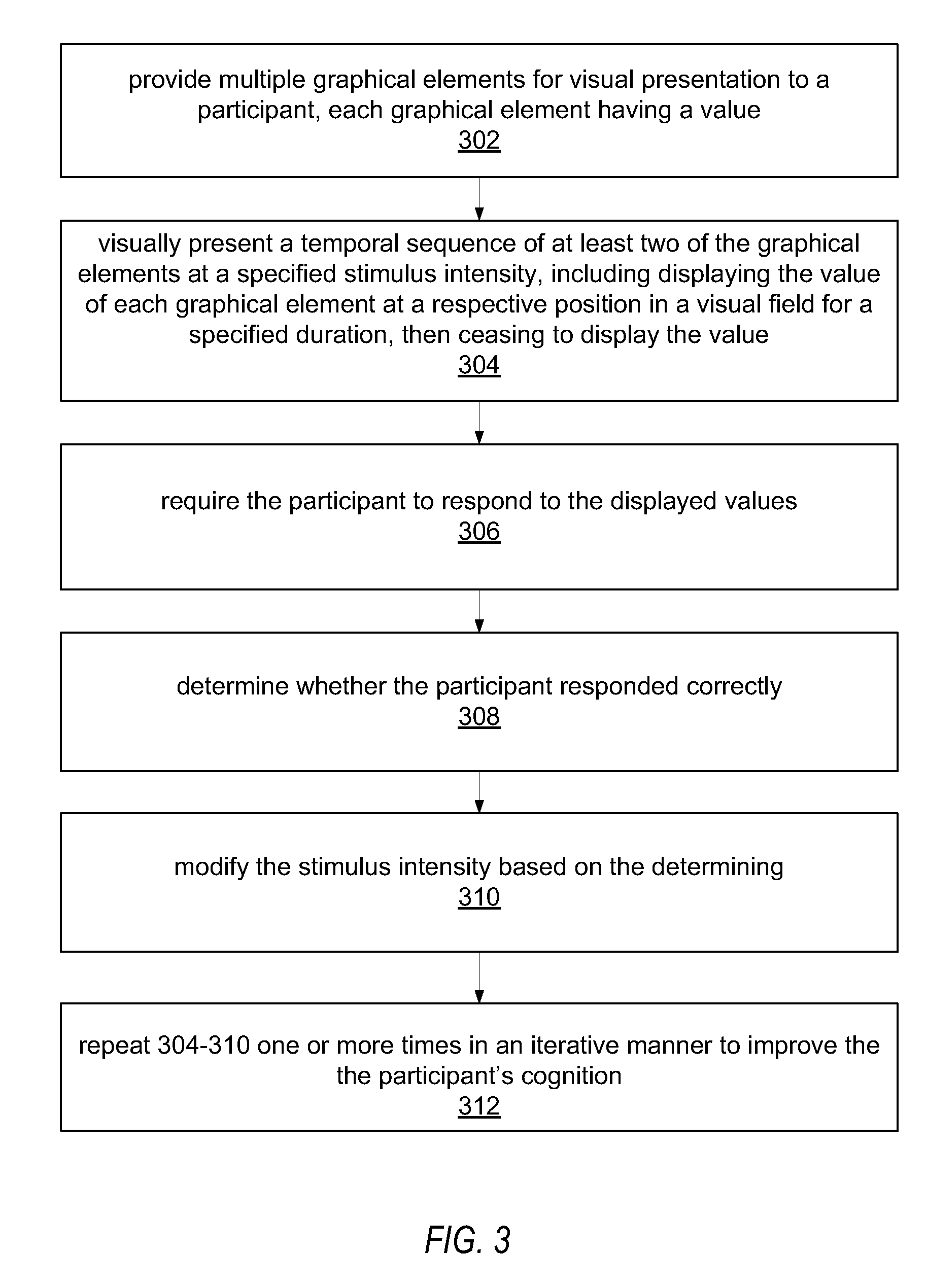
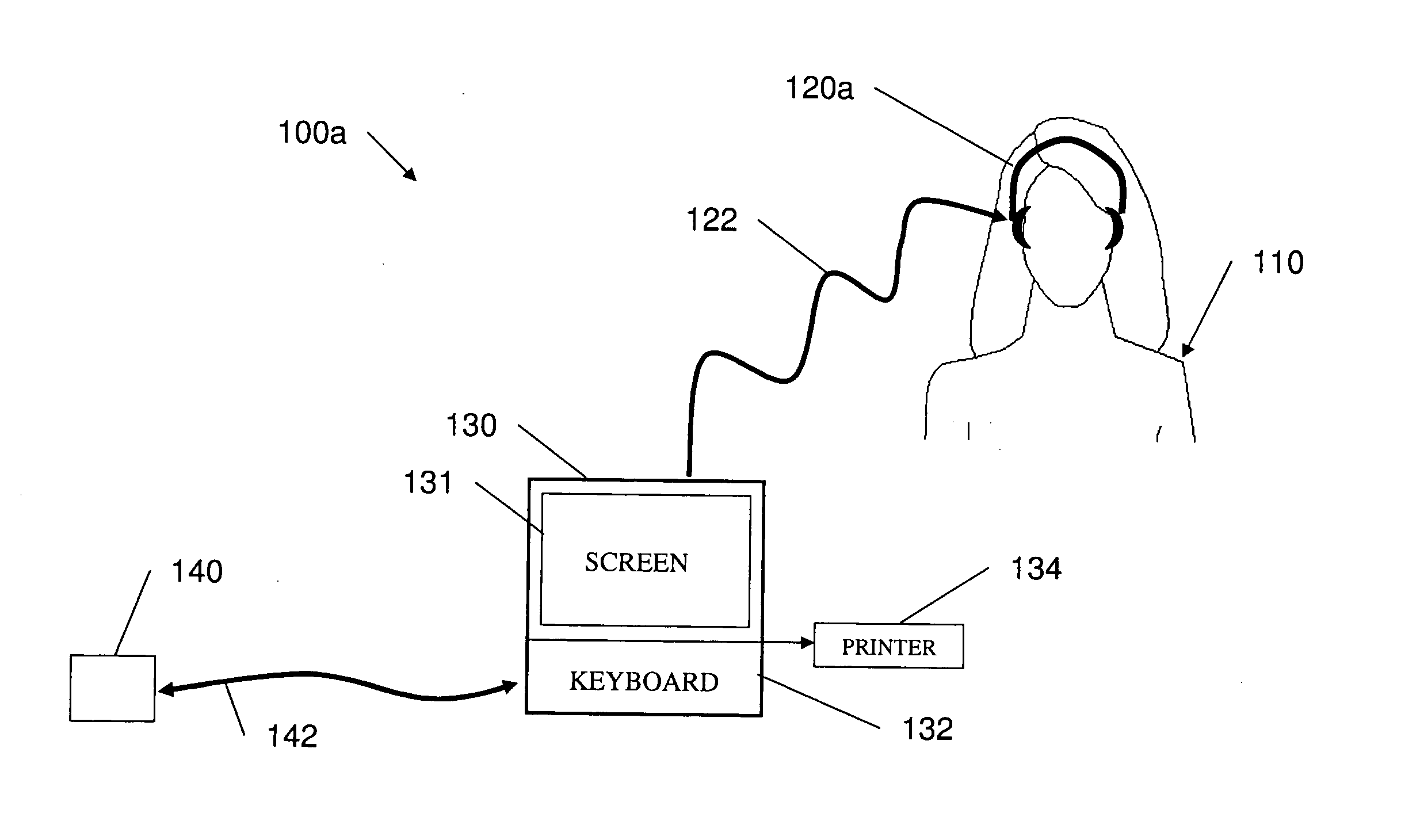
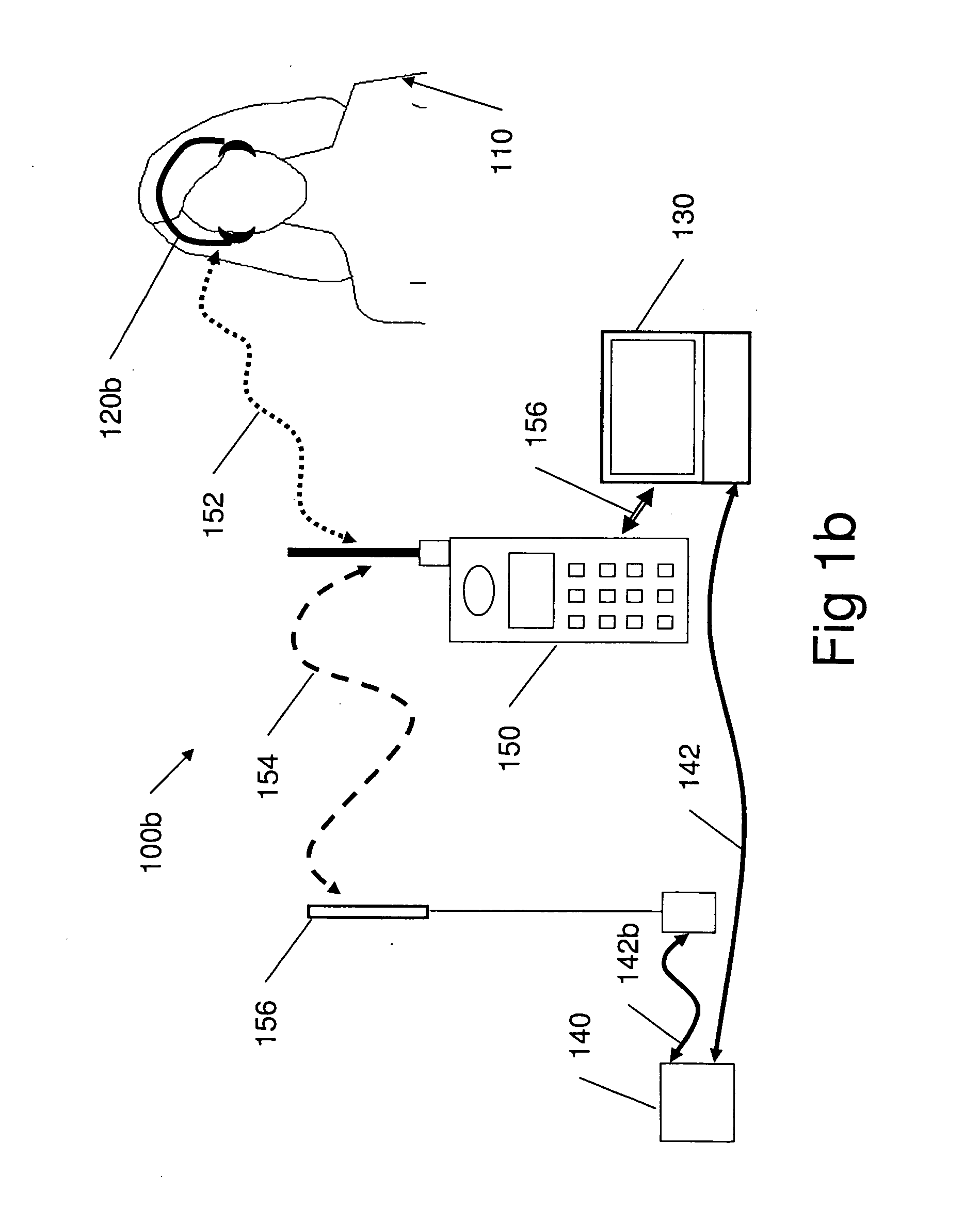
Cognitive training using guided eye movements
InactiveUS20070166676A1Improve cognitionImprove efficiencyElectrical appliancesTeaching apparatusCognitive skillVisual field loss
Computer-implemented method for enhancing cognitive ability of a participant using guided eye movements. Multiple graphical elements are provided for visual presentation to the participant, each having a value. A temporal sequence of at least two of the graphical elements are visually presented at a specified stimulus intensity, e.g., duration or presentation time, including displaying the value of each graphical element at a respective position in a visual field for a specified duration, then ceasing to display the value. The participant is required to respond to the displayed values. A determination is made as to whether the participant responded correctly, and the stimulus intensity modified in response, e.g., using a maximum likelihood procedure. The visually presenting, requiring, determining, and modifying are repeated in an iterative manner to improve the participant's cognitive skills. Periodically, assessments of the participant's progress are performed, e.g., using the maximum likelihood procedure.
Owner:POSIT SCI CORP
Pyrrole and pyrazole DAAO inhibitors
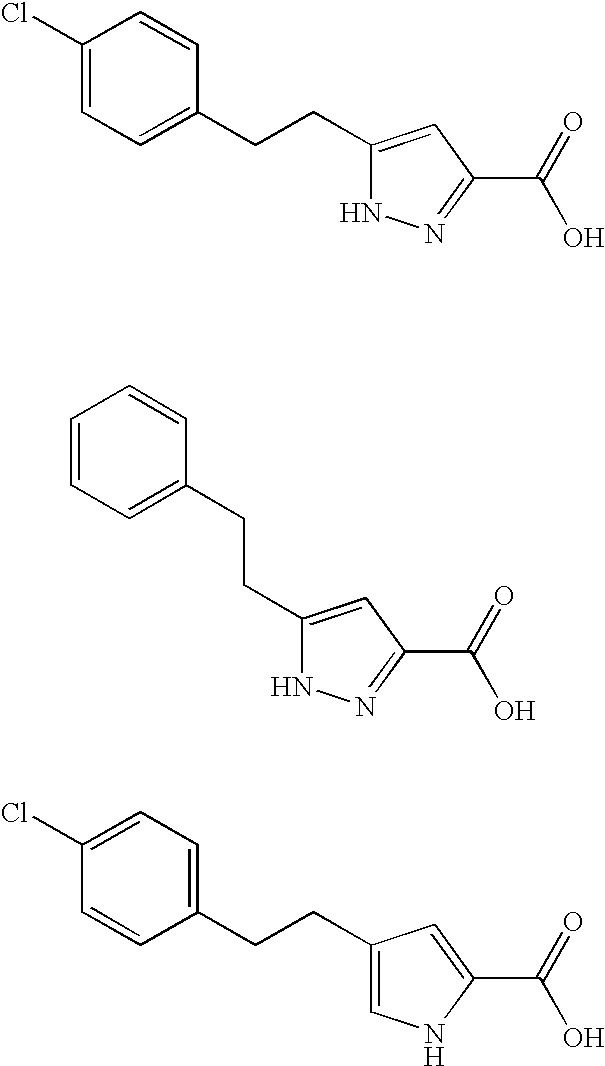
Methods for increasing D-Serine concentration and reducing concentration of the toxic products of D-Serine oxidation, for enhancing learning, memory and / or cognition, or for treating schizophrenia, Alzheimer's disease, ataxia or neuropathic pain, or preventing loss in neuronal function characteristic of neurodegenerative diseases involve administering to a subject in need of treatment a therapeutically effective amount of a compound of formula I, or a pharmaceutically acceptable salt or solvate thereof: wherein [0001]R1 and R2 are independently selected from hydrogen, halo, nitro, alkyl, acyl, alkylaryl, and XYR5; [0002]or R1 and R2, taken together, form a 5, 6, 7 or 8-membered substituted or unsubstituted carbocyclic or heterocyclic group; [0003]X and Y are independently selected from O, S, NH, and (CR6R7)n; [0004]R3 is hydrogen, alkyl or M+; M is aluminum, calcium, lithium, magnesium, potassium, sodium, zinc ion or a mixture thereof; [0005]Z is N or CR4; [0006]R4 is from selected from hydrogen, halo, nitro, alkyl, alkylaryl, and XYR5; [0007]R5 is selected from aryl, substituted aryl, heteroaryl and substituted heteroaryl; [0008]R6 and R7 are independently selected from hydrogen and alkyl; n is an integer from 1 to 6; [0009]at least one of R1, R2 and R4 is other than hydrogen; and [0010]at least one of X and Y is (CR6R7)n. D-serine or cycloserine may be coadministered along with the compound of formula I.
Owner:SEPACOR INC
Auditory diagnosis and training system apparatus and method
InactiveUS20110313315A1Improve cognitionImprove intelligibilityLocal control/monitoringAudiometeringAuditory systemData transmission
The present invention provides a system and method for auditory skills Improvement and, more specifically, for improving auditory perception using a system and method that screens, diagnoses and trains the auditory system. The system is compatible for hearing aid users and for children. The training is specific to the auditory profile of the patients with different task difficulties. To monitor and control the training, the system transfer specific data to a remote server.
Owner:CARMEL HAIFA UNIV ECONOMIC
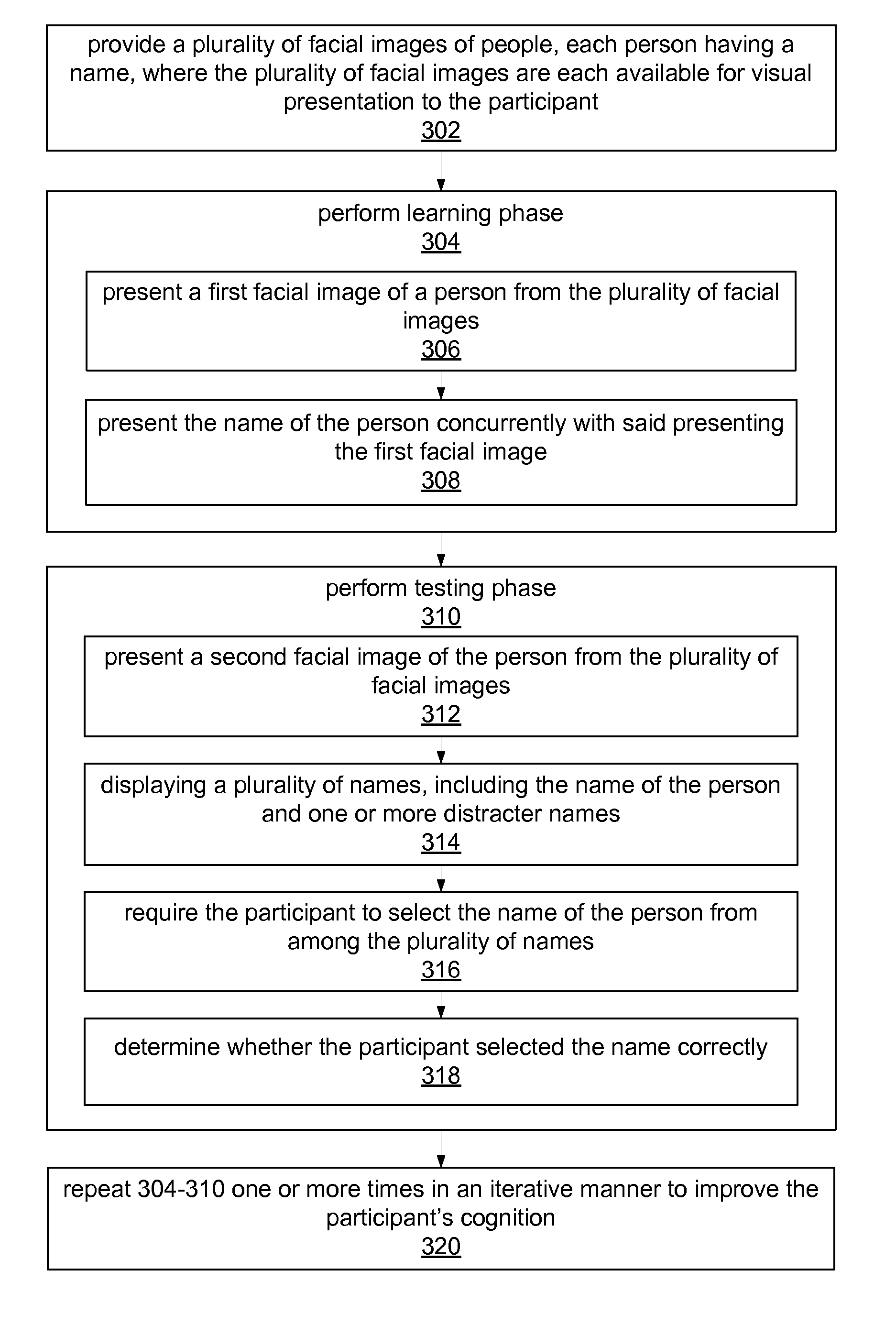
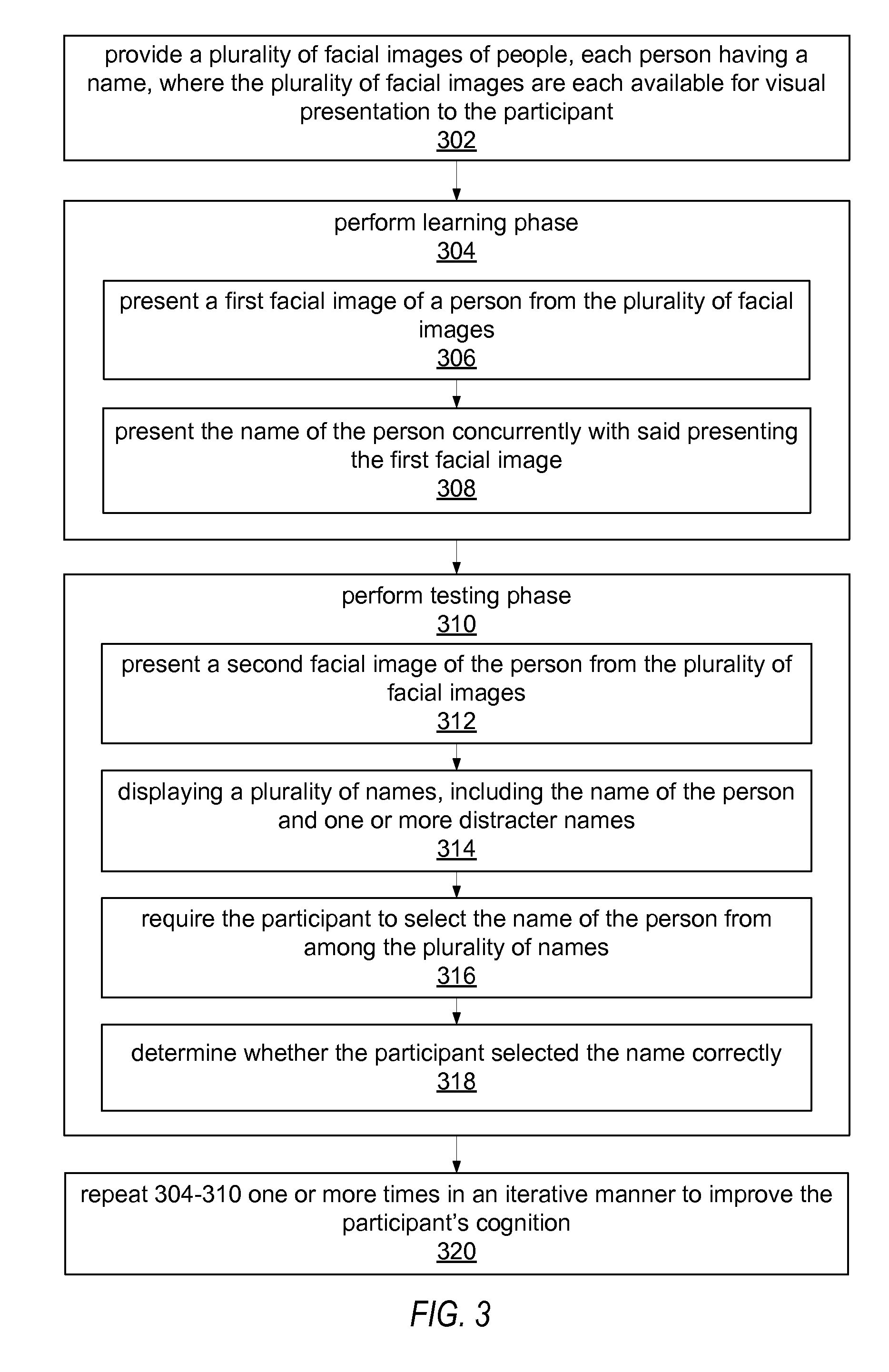
Cognitive training using face-name associations
ActiveUS20070218441A1Strengthen associationIncrease awarenessMental therapiesElectrical appliancesVisual presentationPhysical medicine and rehabilitation
Computer-implemented method for enhancing the cognitive ability of a participant using face-name associations. A plurality of facial images of people are provided for visual presentation to the participant, each person having a name. A learning phase is performed, including concurrently presenting a first facial image of a person from the plurality of facial images, and the name of the person. A testing phase is then performed, including: presenting a second facial image of the person from the plurality of facial images, displaying a plurality of names, including the name of the person and one or more distracter names, requiring the participant to select the name of the person from the plurality of names, and determining whether the participant selected the name correctly. The learning phase and the testing phase are repeated one or more times in an iterative manner to improve the participant's cognition, e.g., face-name association skills.
Owner:POSIT SCI CORP
Peptides containing tryptophan
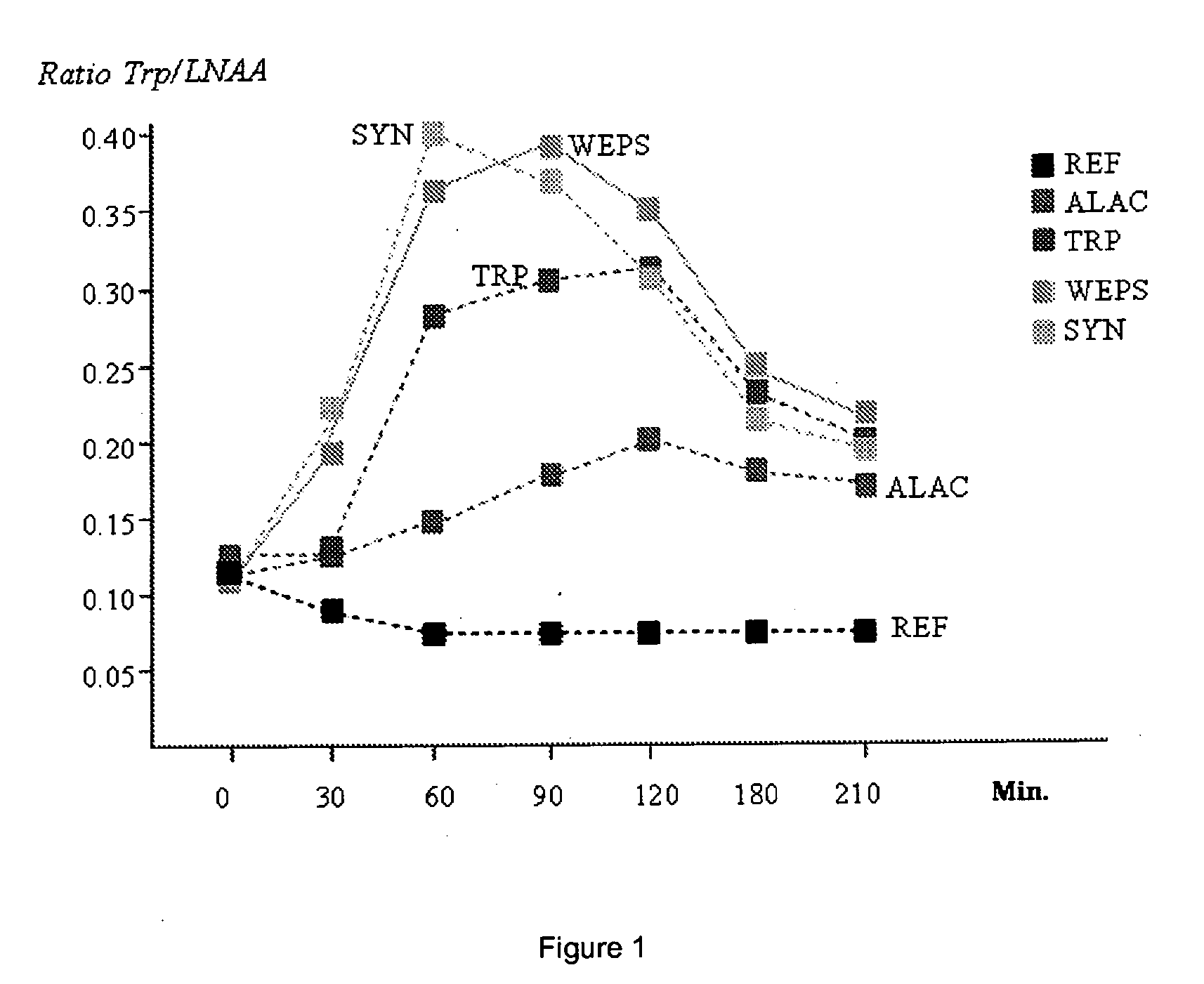
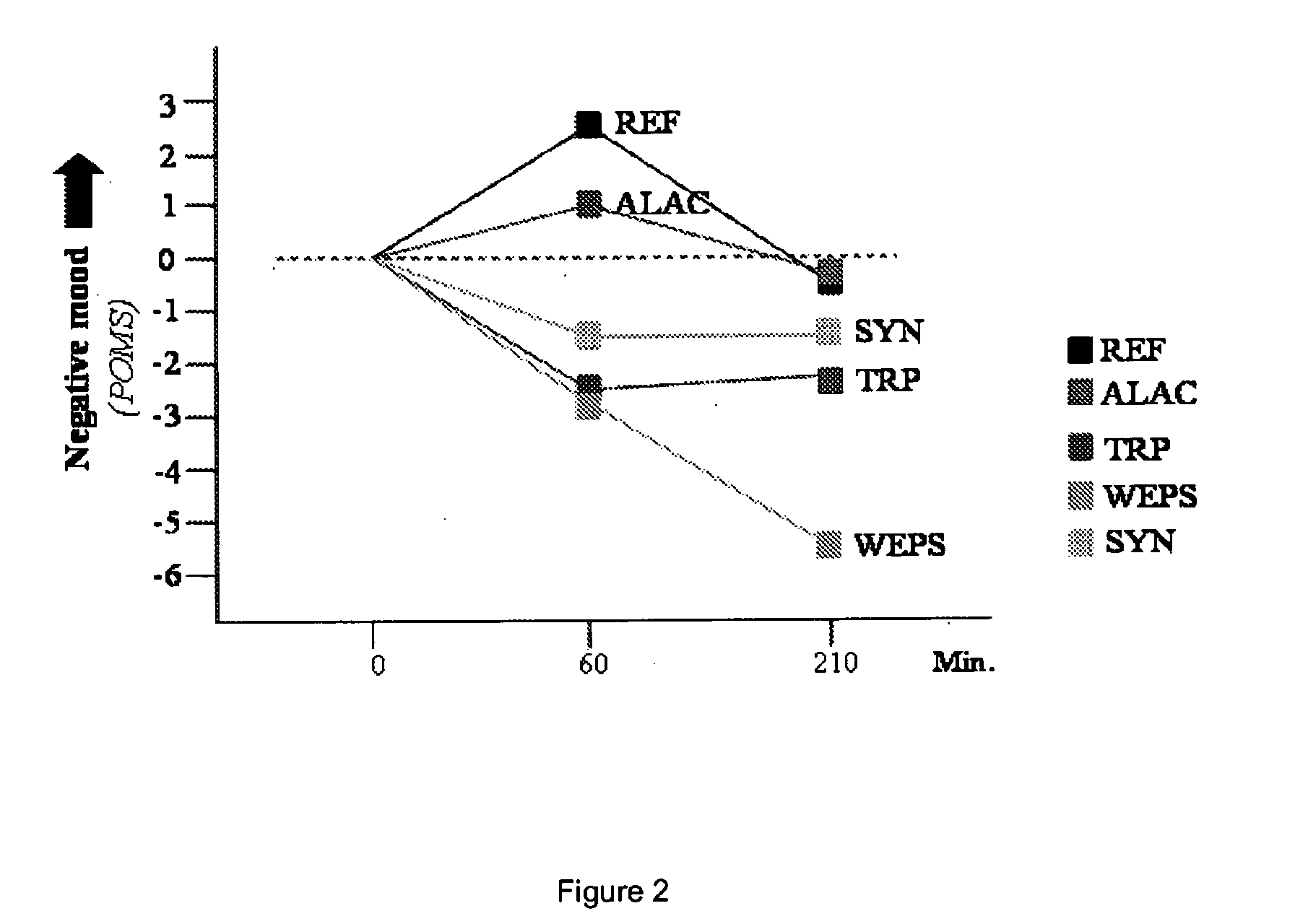
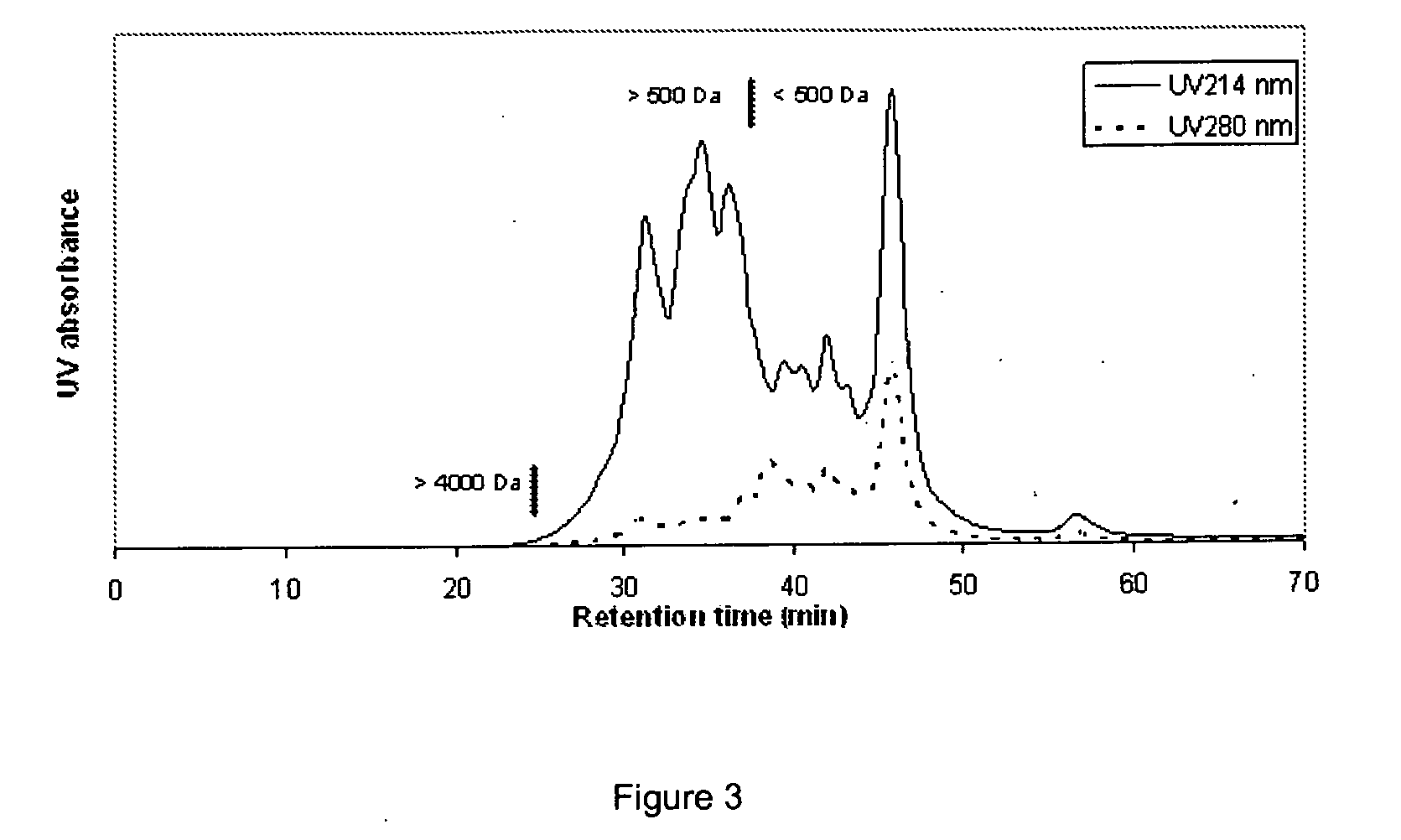
InactiveUS20110086803A1Relieve stressIncreasing Trp/LNAA ratioBiocideNervous disorderHydrolysateHen Egg Lysozyme
The present invention relates to a process to produce a composition comprising water-soluble peptides and having a Trp / LNAA ratio of more than 0.15, which comprises hydrolyzing lysozyme, preferably hen eggs lysozyme, to prepare a hydrolysate having a DH of between 5 and 45.
Owner:DSM IP ASSETS BV
Nutritional compositions including rrr-alpha tocopherol and polyunsaturated fatty acids
InactiveUS20150025133A1Improved central nervous system maturationImproved cognitive developmentBiocideSugar food ingredientsBrain developmentIodo fatty acid
Disclosed are nutritional formulas generally, and infant formulas specifically, including a combination of RRR-alpha tocopherol, LC-PUFAs, and optionally vitamin C. The combination enhances brain development and improves cognitive performance in an individual, and specifically in an infant.
Owner:ABBOTT LAB INC
Therapies for cognition and learning enhancement
InactiveUS20090252704A1Easy to learnImprove cognitionBiocidePeptide/protein ingredientsDiseaseCognition
The invention relates to a combination comprising an amount of an NO donor, such as ISDN, and / or an amount of another pharmaceutical agent that enhances neurotransmission or which acts as neuroprotectants such as memantine, clomethiazole and tacrine. These compositions can be used in producing cognition and learning enhancement, whereby the invention also provides for a new method of treatment of Alzheimer's disease and related neurodegenerative disorders.
Owner:GREEN ALLAN M +1
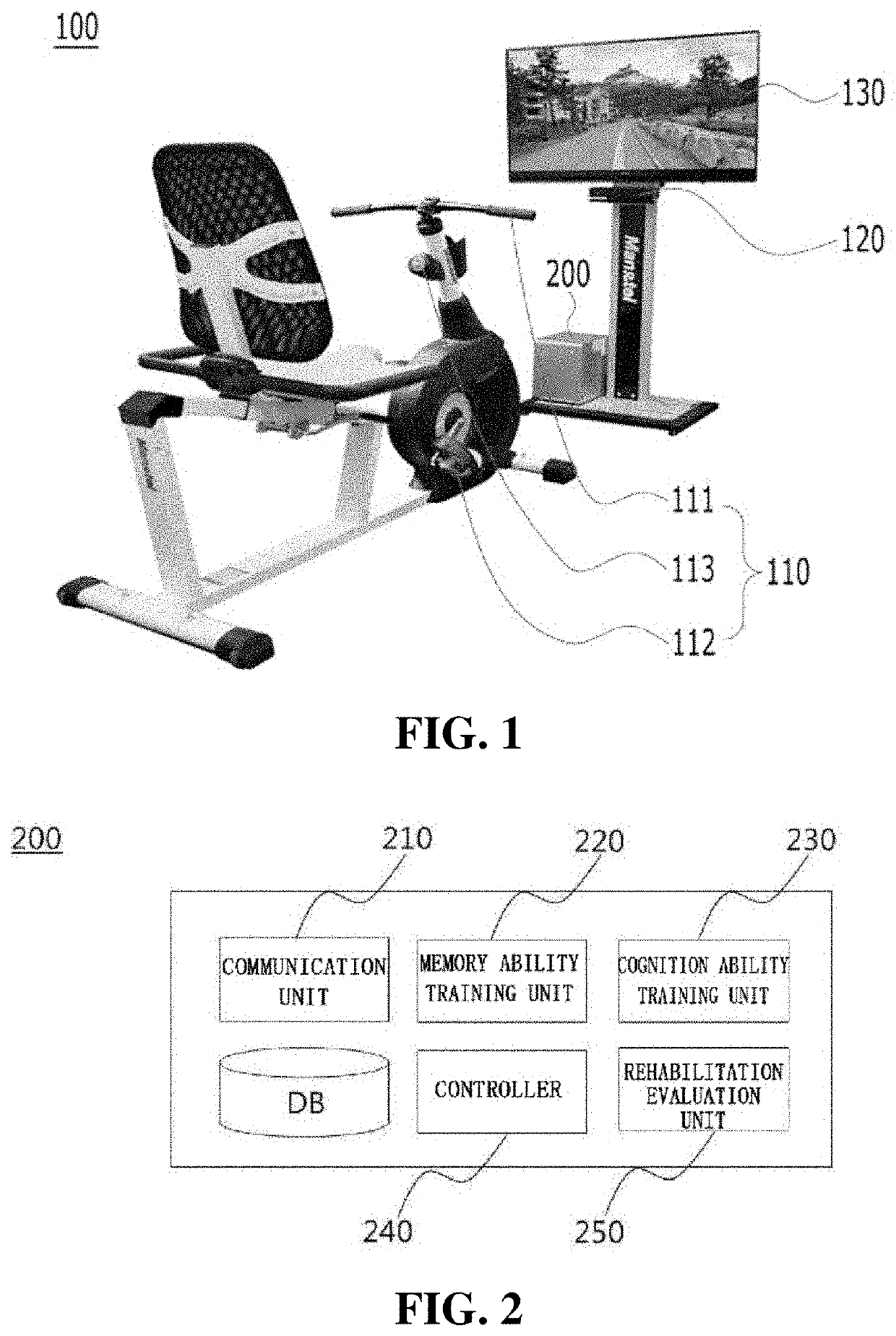
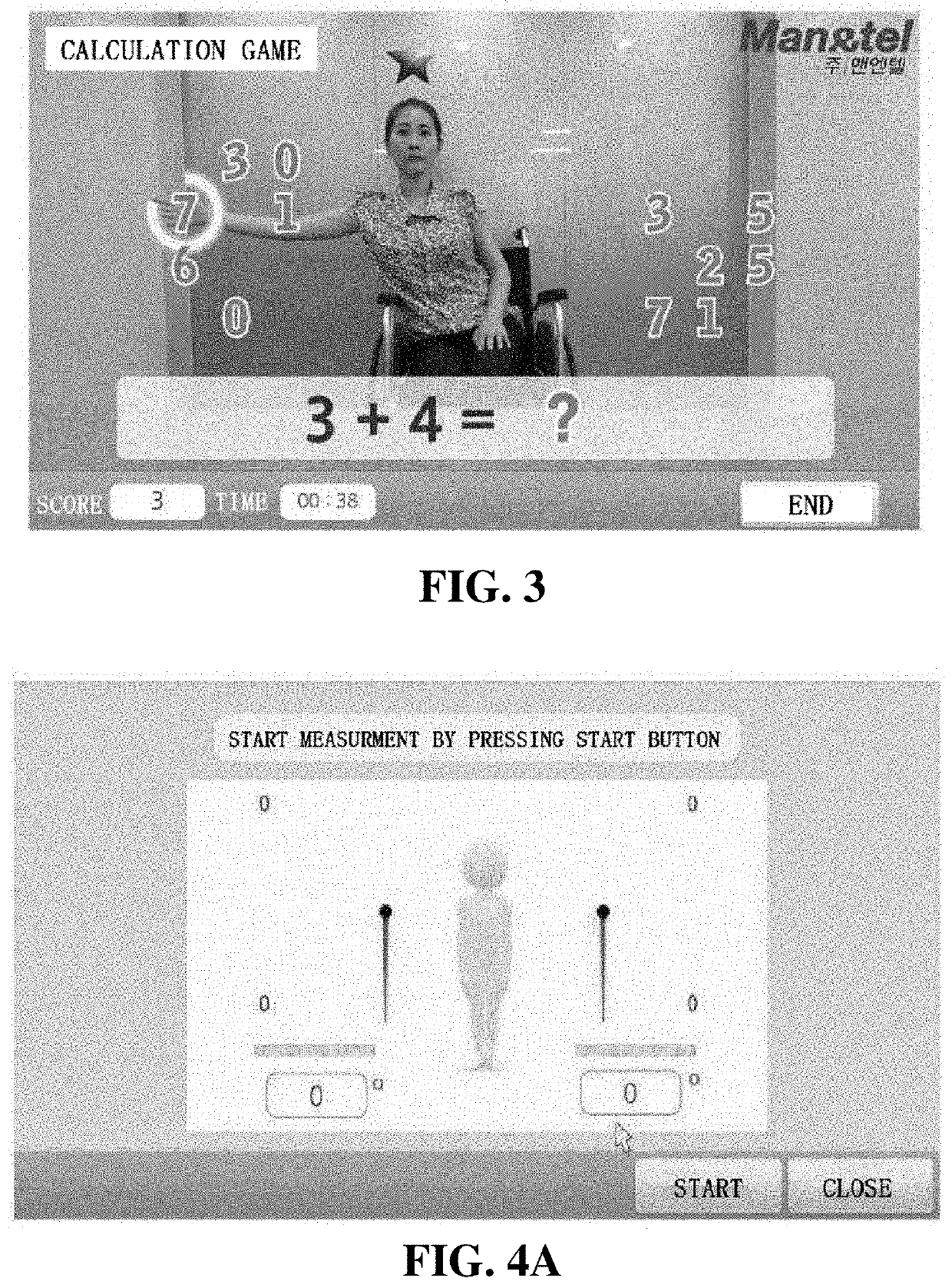
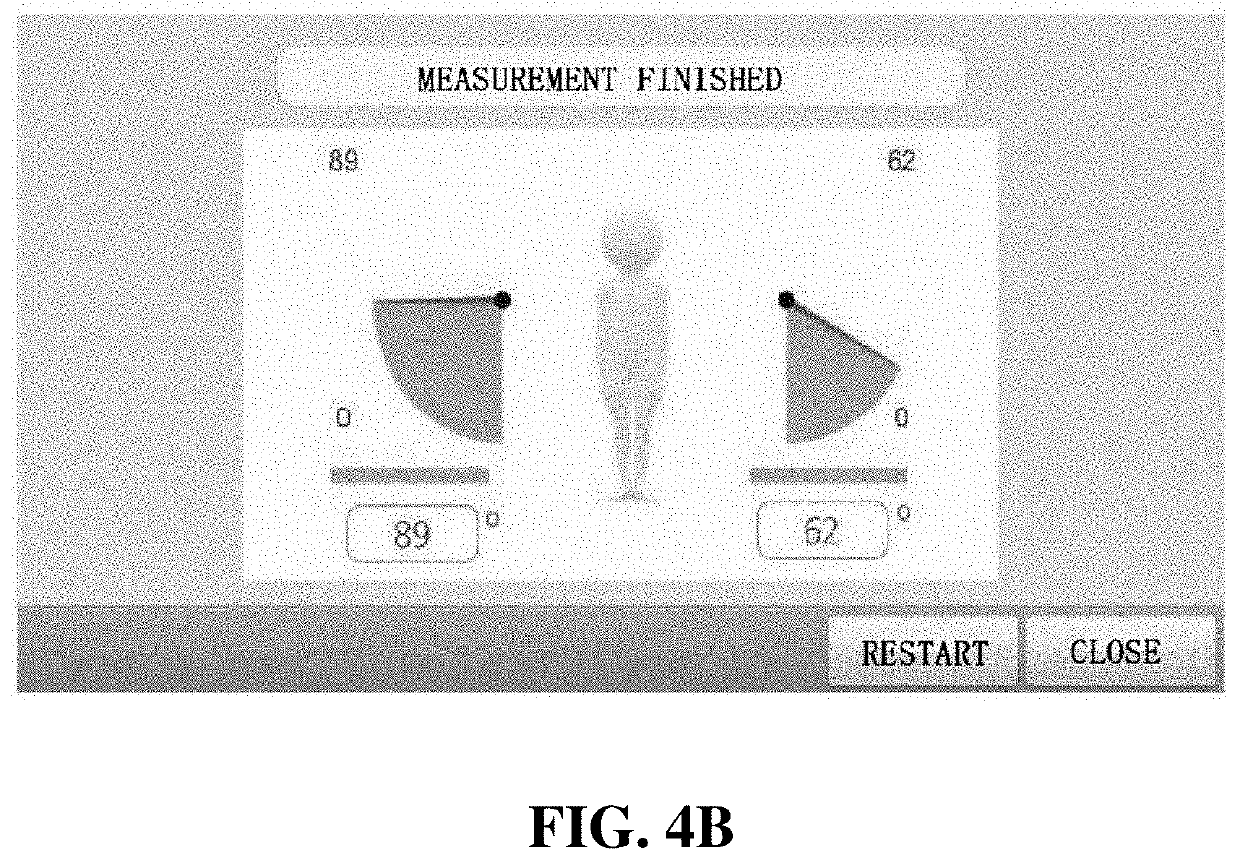
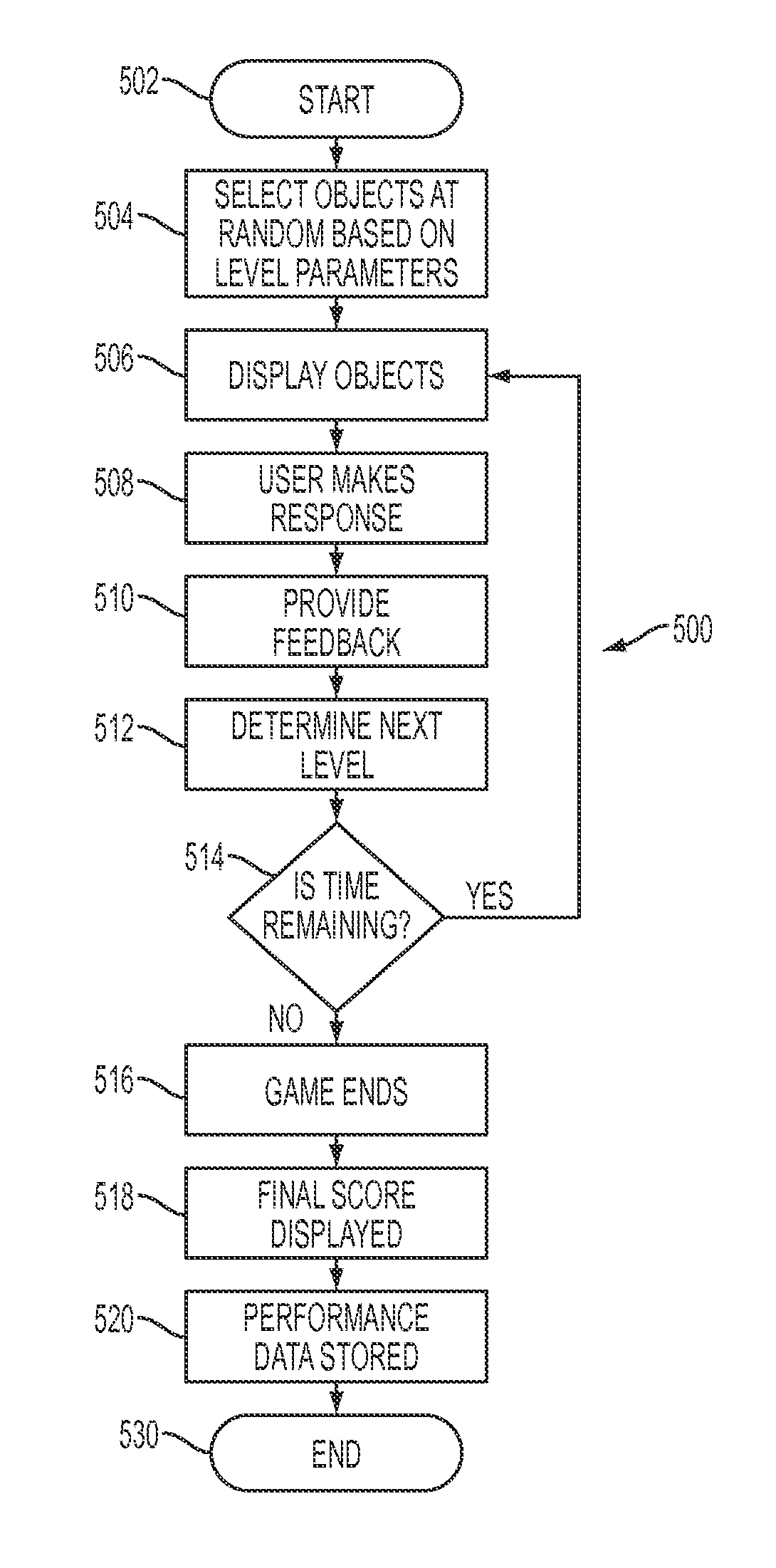
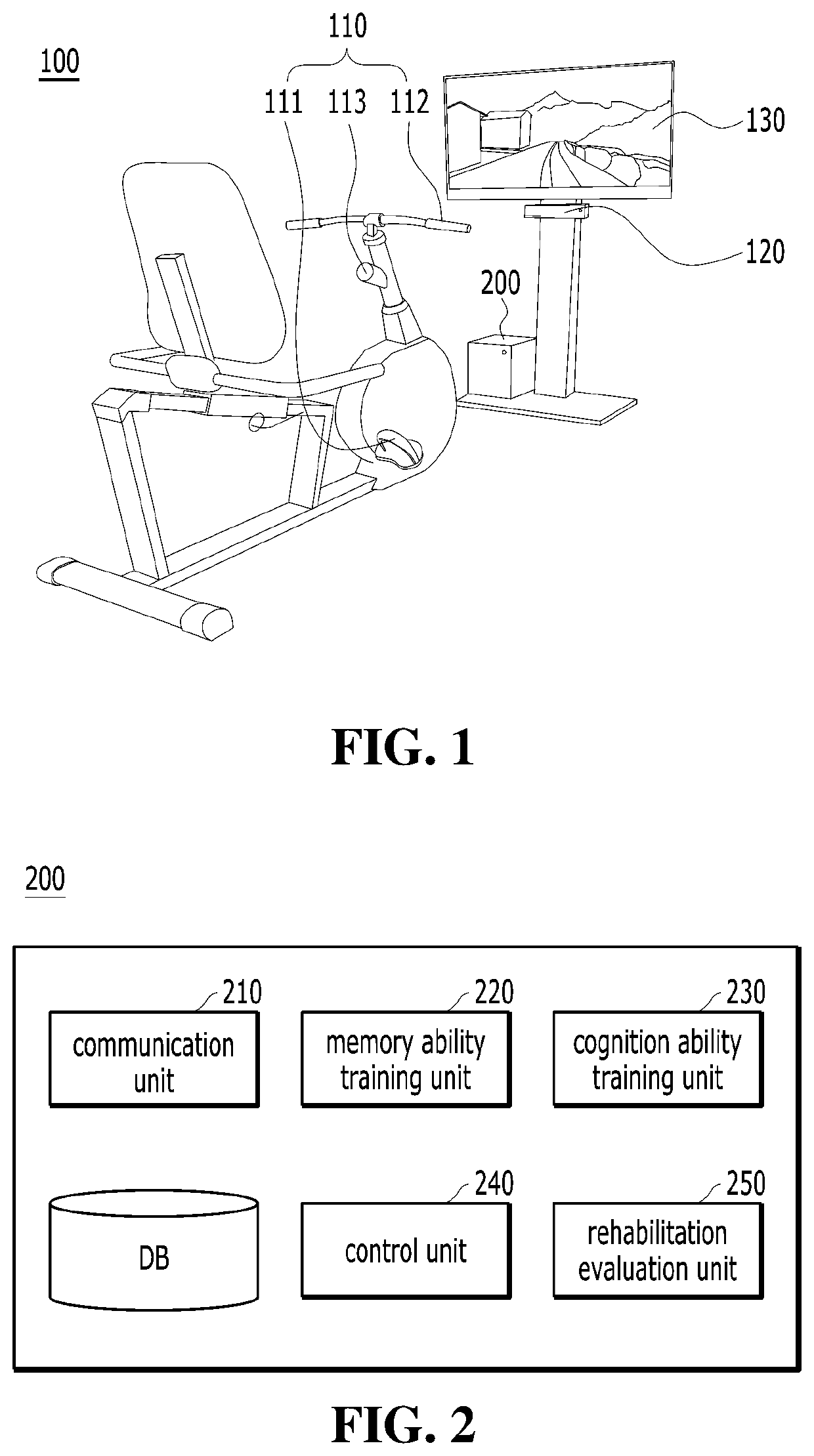
Training equipment to improve the ability of cognition and memory and the muscle power of upper and lower limb and training method thereof
ActiveUS20200206567A1Increase muscle strengthLow powerVideo gamesTeaching apparatusUpper limb muscleVirtual space
The present invention relates to training equipment for improving the ability of memory and cognition, and muscle power, and a training method using the training equipment. In particular, the present invention relates to training equipment for improving the ability of memory and cognition, and muscle power (upper and lower limb muscle power), the training equipment including: a lower limb muscle power exercise module that includes pedals for strengthening the muscle power of user's lower limbs and / or a handle for steering; a display that implements traveling in a virtual space for memory ability training of the user; and a training unit that improves cognition ability and / or muscle power of the user on the basis of driving data of the lower limb muscle power exercise module, thereby being able to complexly support memory ability training, lower limb muscle power exercise, and upper limb muscle power exercise of rehabilitation patients, patients with dementia, and / or patients with cognitive impairment.
Owner:MAN&TEL
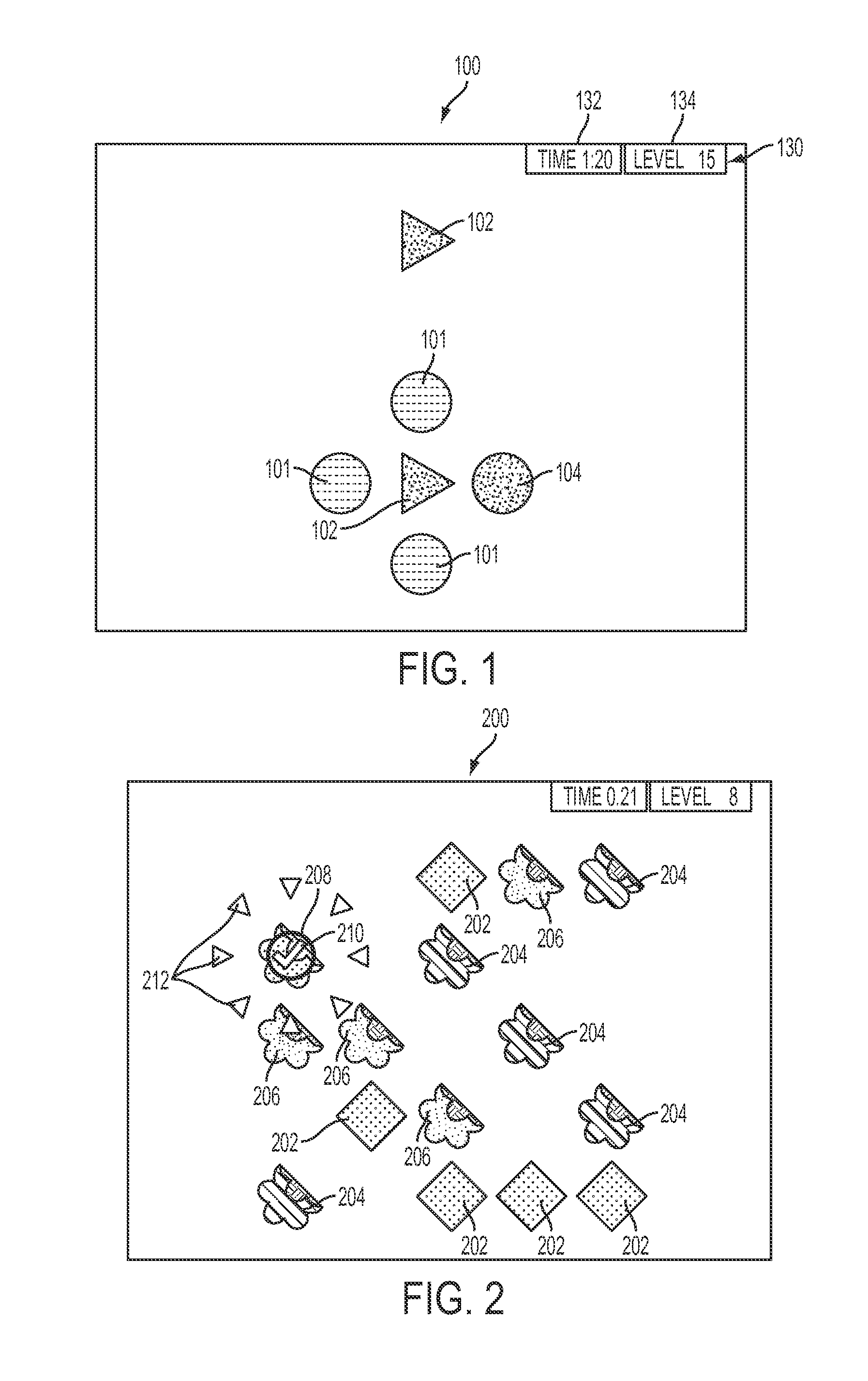
Systems and methods for a search driven, visual attention task for enhancing cognition
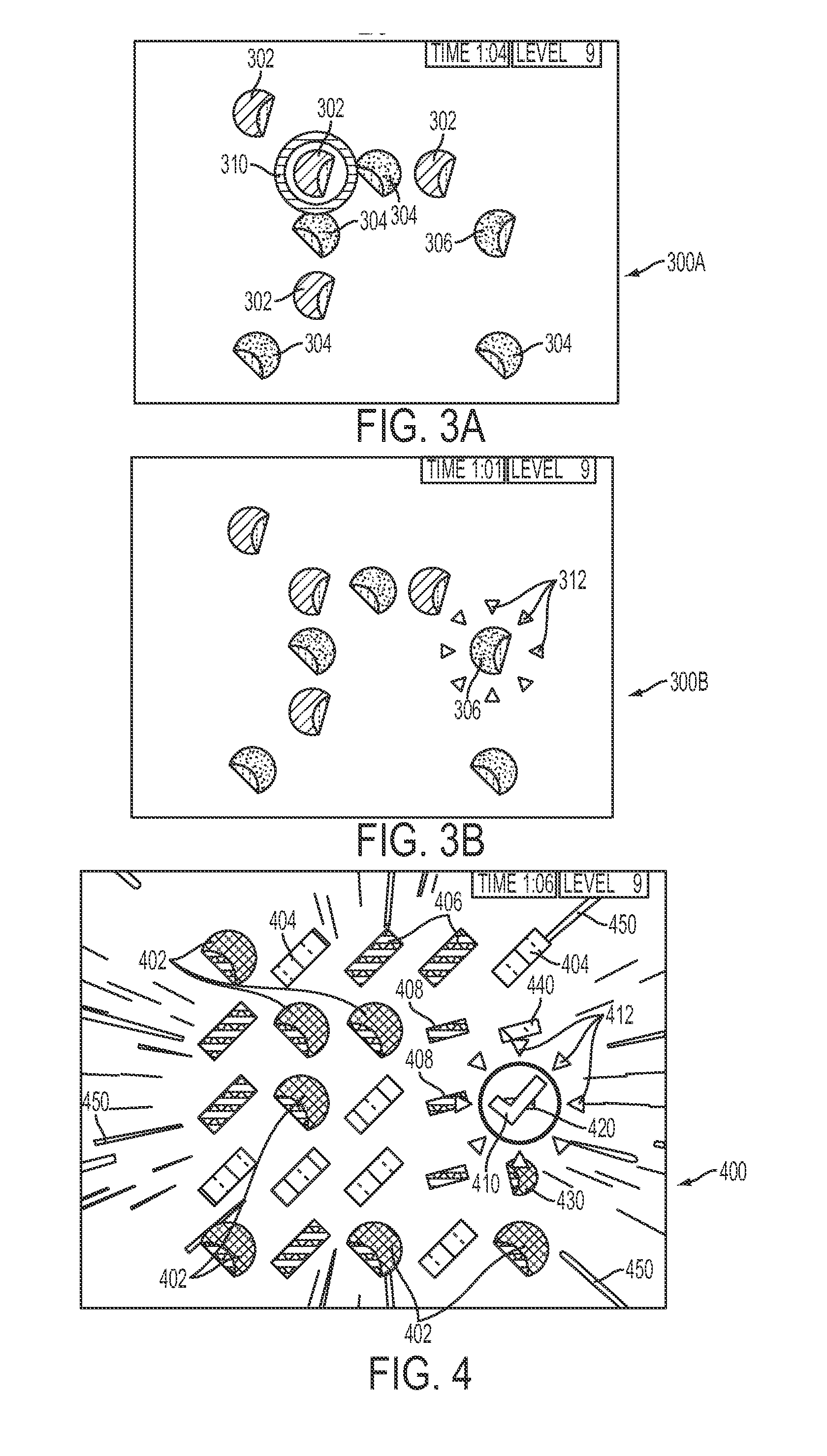
InactiveUS20150093730A1Improve cognitionImprove skillsElectrical appliancesTeaching apparatusDisplay deviceUnique object
A method and apparatus for enhancing a cognitive ability of a user is disclosed, which may comprise: conducting, via a user interface display of a user computing device, a training session which may comprise: presenting, via the user interface display of the user computing device, a plurality of objects having at least two identifying parameters, at least two of the plurality of objects having the same at least two identifying parameters forming at least one group of objects having the same at least two identifying parameters and at least one of the objects having at least one unique identifying parameter thereby not duplicating the identifying parameters of the at least two objects in the at least one group of objects; and allowing the user, via the user interface display of the user computing device, to select an object as a proposed unique object.
Owner:LUMOS LABS
Decision-making system using emotion and cognition inputs
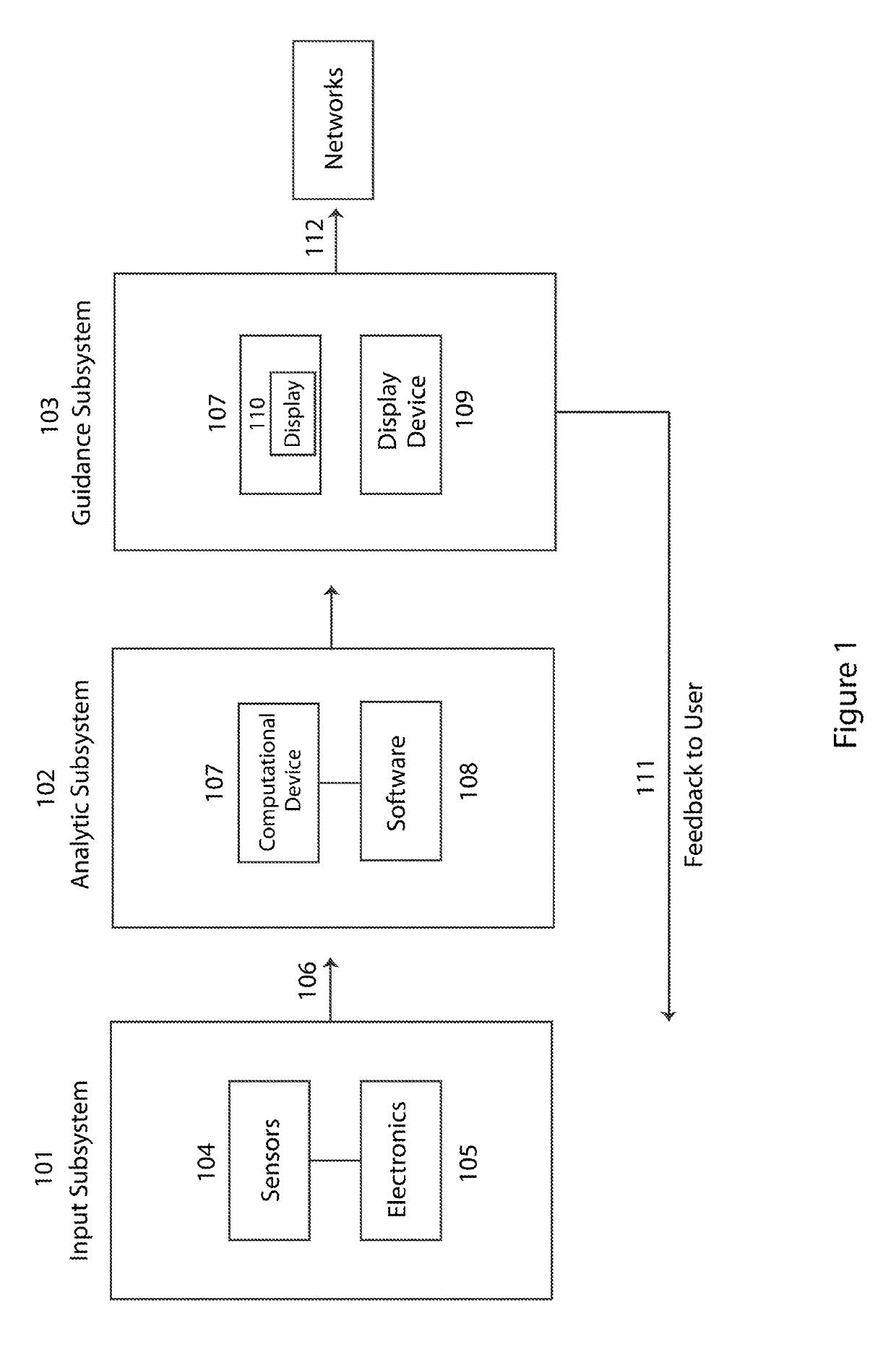
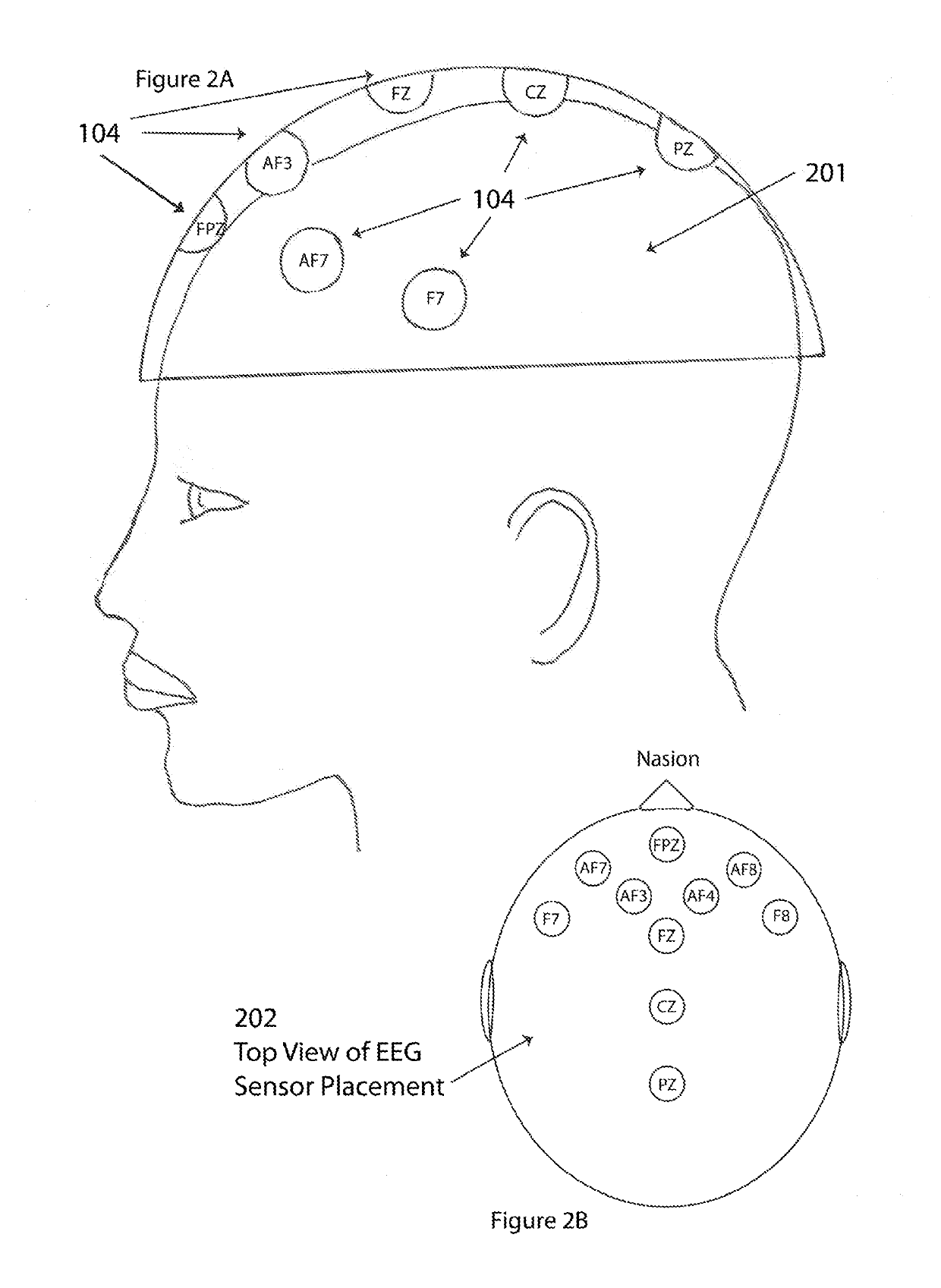
InactiveUS20190096279A1Improve cognitionIncreased memory utilization effortElectrical appliancesTeaching apparatusCognitionEngineering
A method and system for assessing an influence of emotion and cognition components on a decision-making process includes positioning a plurality of sensors with respect to a person and using the plurality of sensors to measure a physiologic condition of the person. A computational device quantifies one or more parameters representative of an emotion state and a cognition or memory utilization effort of the person before and during a decision-making process based on a sensed physiologic condition of the person, and further determines a relative influence of emotion versus cognition or memory utilization effort in the decision-making process based on the one or more quantified parameters representative of the emotion state and cognition or memory utilization effort of the person. The sensors may be attached to or embedded in a headgear or headset that is placed on the person's head to measure the physiologic condition of the person.
Owner:LAU MICHAEL P H
Bioactive alkaloid compositions and their medical uses
ActiveUS20150018292A1Increase painReduced joint mobilityBiocideSaccharide with carbocyclic radicalsDyslipidemiaMuscle dysfunction
The present invention relates to a novel alkaloid and novel bioactive alkaloid fractions derivable from Ribes preferably selected among Ribes Rubrum and Ribes nigrum; methods of manufacturing such bioactive Ribes alkaloid fractions and their use for the inhibition of IKK-β, PDE4 and / or PDE5 and in addition their promoting effect on mitochondrial biogenesis and function; their therapeutic or non-therapeutic applications as nutritive or medicinal products in the management of conditions associated with impaired mitochondrial function or IKK-β, PDE4 and / or PDE5 activity, such as inflammation, neurodegeneration, dyslipidemia, type 2 diabetes mellitus, impaired wound healing, sarcopenia and other conditions associated with muscle dysfunction or tiredness and fatigue, or where optimization of muscular or cognitive function is desired; extracts, juices or concentrates of Ribes comprising such alkaloids; compostions comprising such alkaloids, including pharmaceutical compositions, nutritive product such as functional foods and nutraceutical compositions, and cosmetic compositions and medical devices.
Owner:ASIROS
Novel use for pde5 inhibitors
InactiveUS20070185114A1Increase blood perfusionRaise attentionAntibacterial agentsBiocidePhosphodiesterase 5 inhibitorPharmacology
Owner:TAKEDA GMBH
Methods and Compositions for Treating Aging-Associated Impairments Using CCR3-Inhibitors
ActiveUS20190105314A1Improve cognitionImproved motor activityPowder deliveryOrganic active ingredientsHuntingtons choreaMyotonic dystrophy gene
Methods of improving neurodegenerative disease with CCR3 modulating agents are provided. The methods include administering a therapeutically effective amount of the CCR3 modulating agent to the subject, with a concomitant improvement in cognition, motor, or other neurodegenerative-affected function. Cognitive and motor diseases upon which the methods of the invention can improve cognition include Alzheimer's disease, Parkinson's disease, frontotemporal dementia, Huntington's disease, amyotrophic lateral sclerosis, multiple sclerosis, glaucoma, myotonic dystrophy, vascular dementia, progressive supranuclear palsy.
Owner:ALKAHEST INC
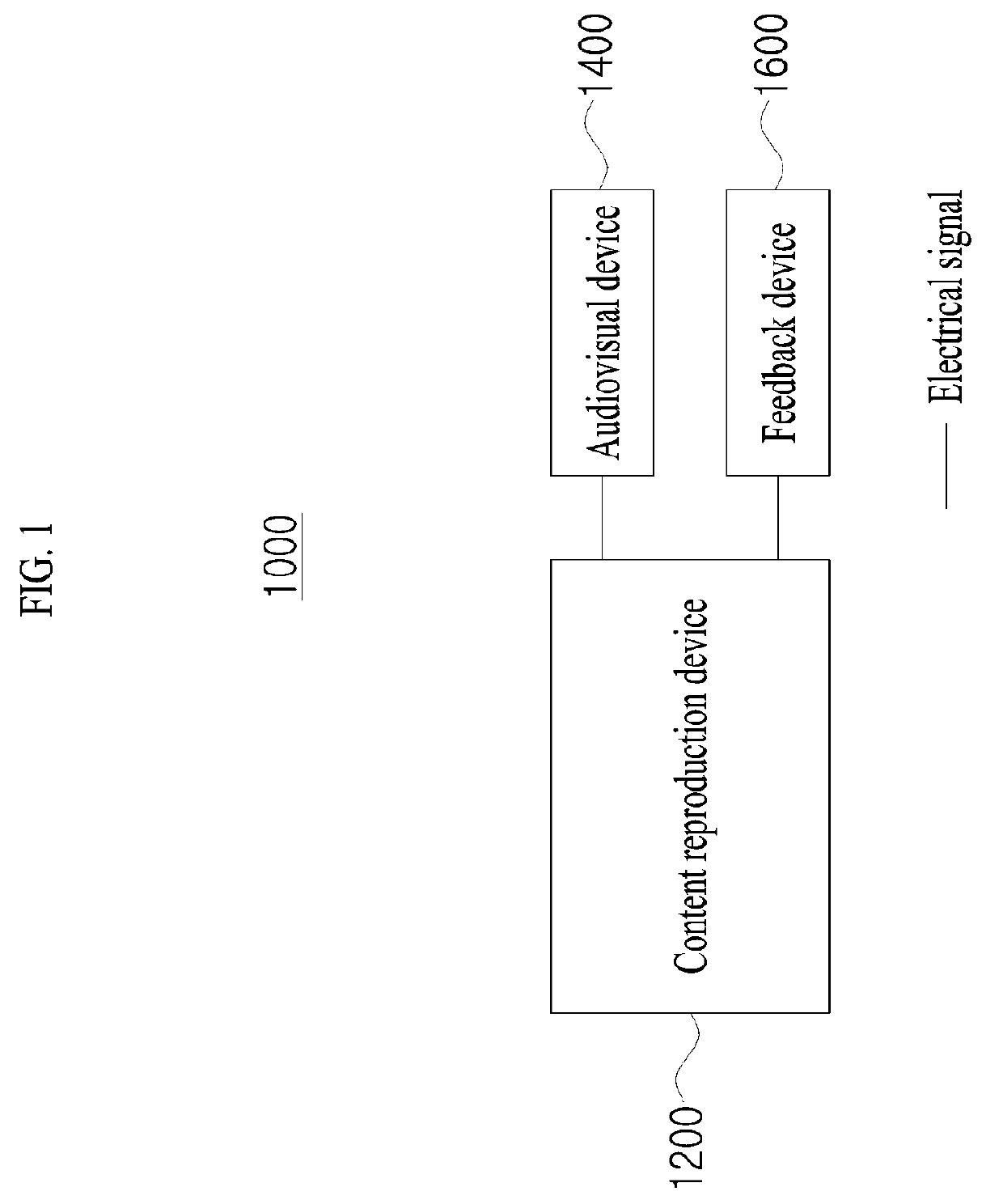
Feedback device and method for providing thermal feedback by means of same
ActiveUS20190250710A1Provide feedbackImprove cognitionInput/output for user-computer interactionThermoelectric device with peltier/seeback effectThermal feedbackEngineering
The present invention relates to a feedback device and a thermal feedback provision method using the same. The thermal feedback provision method may include checking first operating power applied to a first thermoelectric couple group for a first thermoelectric operation and second operating power applied to a second thermoelectric couple group for a second thermoelectric operation when the first thermoelectric operation is initiated in the first thermoelectric couple group to initiate the output of the first thermal feedback after the second thermoelectric operation is initiated in the second thermoelectric couple group to initiate the output of the second thermal feedback and include applying cognitive enhancement power for enhancing a user's cognition to the first thermoelectric couple group from a time point at which the output of the first thermal feedback is initiated up to a first time point so that the user's cognition of the first thermal feedback is enhanced.
Owner:TEGWAY CO LTD
Medium chain fatty acid esters of beta-hydroxybutyrate and butanediol and compositions and methods for using same
ActiveUS20190359551A1Increase awarenessReduce expressionSenses disorderNervous disorderButanediolMedium chain fatty acid
Aspects of the present disclosure include fatty acid β-hydroxyester compounds (e.g., fatty acid esters of β-hydroxybutyrate), fatty acid esters of butanediol, and pharmaceutically acceptable salts thereof. Also provided are pharmaceutical compositions having one or more fatty acid β-hydroxyester compounds and / or one or more fatty acid esters of butanediol. Methods for treating a subject by administering one or more esters to the subject are also provided. Kits containing one or more of the subject esters are also described.
Owner:RGT UNIV OF CALIFORNIA +2
Peptides containing tryptophan
ActiveUS20130231278A1Raise the ratioIncrease alertnessNervous disorderDipeptide ingredientsHydrolysateWater soluble
The present invention relates to a process to produce a composition comprising water-soluble peptides and having a Trp / LNAA ratio of more than 0.15, which comprises hydrolyzing lysozyme, preferably hen eggs lysozyme, to prepare a hydrolysate having a DH of between 5 and 45.
Owner:DSM IP ASSETS BV
Emotional regulating system for Chinese old people and method
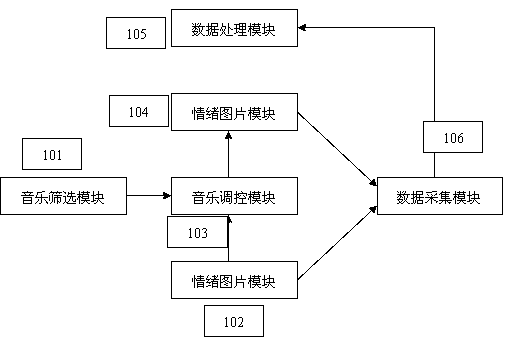
InactiveCN102908710AImprove cognitionData processing applicationsSleep inducing/ending devicesOlder peopleData acquisition
The invention discloses an emotional regulating system for Chinese old people and a method. The system comprises a music screening module for selecting emotional music by a questionnaire method to record and manage the emotional music and a emotional experience score, an emotional image module for providing emotional image models for emotional excitation and maintenance, a music regulating module which is a main component for the emotional regulation for emotional excitation and strengthening, a data acquisition module for acquiring behavior data before and after music nursing under the emotional image models, and a data processing module for statistic analysis of the behavior data.
Owner:SHANGHAI UNIV
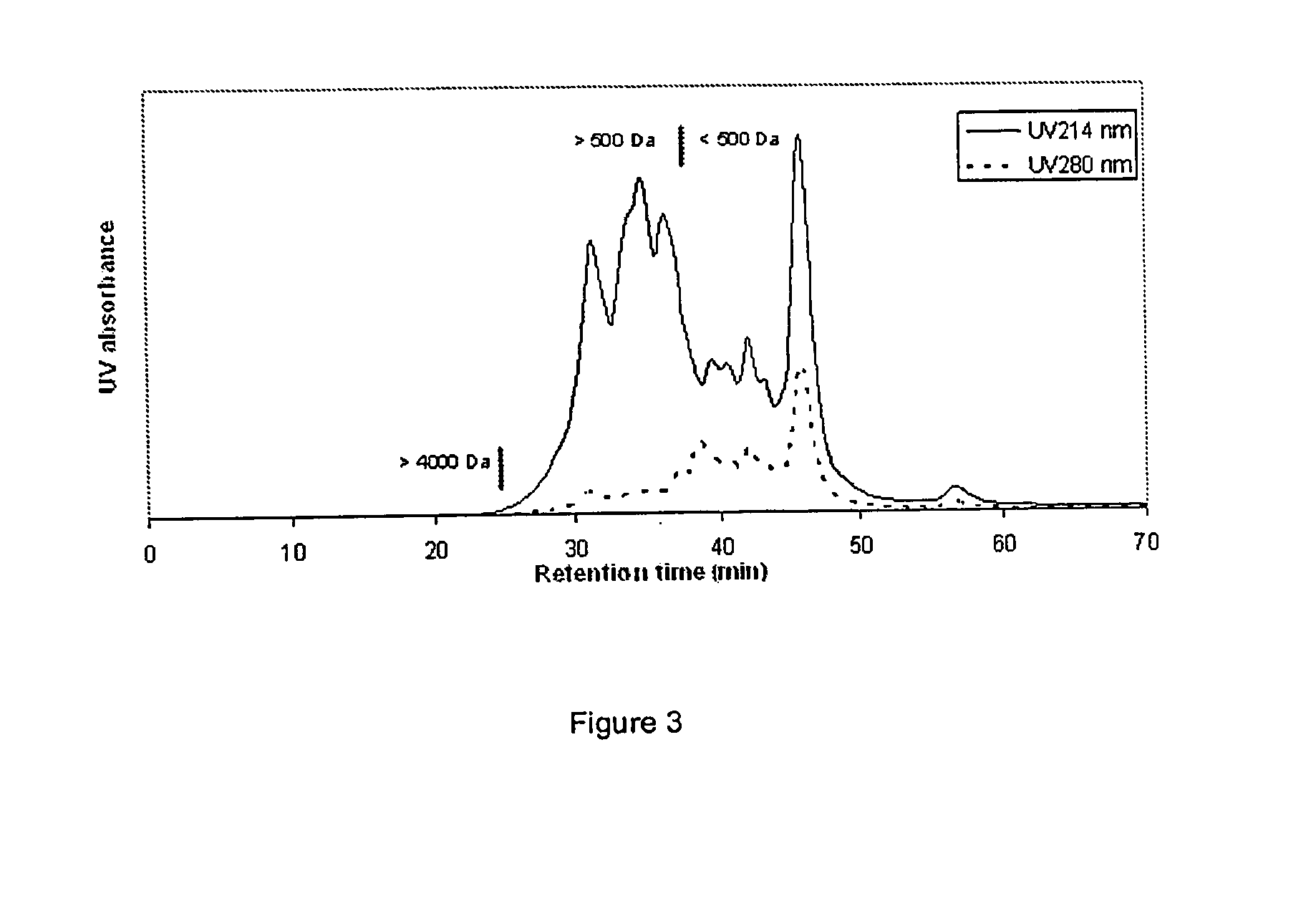
Method for quantitatively detecting content of intermediate in production process of recombinant human interferon alpha by affinity chromatography,
PendingCN109307771AEasy to operateImproved awareness and controlPeptide preparation methodsBiological testingAmmonium sulfateChemistry
The invention relates to a method for quantitatively detecting the content of an intermediate in the production process of recombinant human interferon alpha by affinity chromatography. The method forquantitatively detecting the content of the intermediate in the production process of the recombinant human interferon alpha by affinity chromatography comprises the following steps that (1) engineering bacteria expressing the recombinant human interferon alpha is constructed; and (2) the engineering bacteria is fermented, and the target protein expressed in the form of soluble protein or an inclusion body is separated and purified, wherein the protein purification comprises crude purification and refined purification, a crude protein is obtained by ammonium sulfate salting-out precipitation,the crude protein is purified by DEAE anion exchange column chromatography to obtain the intermediate, the content of the recombinant human interferon alpha in the intermediate is quantitatively detected by a monoclonal antibody affinity chromatography column. The method is suitable for quantitatively detecting the intermediate in the production process of recombinant human interferon alpha and recombinant integrated interferon, and has the advantages of simple, rapid and accurate operation, and can improve the cognition degree and control degree of people on the production process accordingto the obtained interferon concentration data, so that reliable, rapid, direct and easy evaluation of key links of interferon production is achieved so as to realize the purpose of accurate quality control.
Owner:BEIJING TRI PRIME GENE PHARMA CO LTD
Brain health formulation
PendingUS20200316013A1Improve and maintain memoryImprove and maintain and cognitionHydroxy compound active ingredientsPharmaceutical non-active ingredientsSpearmint extractFilm-forming agent
The invention relates to a unique formulation for brain health. The formulation includes a mixture of CBD and THC and / or flavonoids, along with at least one antioxidant, and preferably an emulsifier, spearmint extract and a film-forming agent. The formulation improves memory and cognition as well as prevents and improves symptoms in dementia and related diseases related to deterioration or loss of memory and / or cognition.
Owner:MEDPHARM HLDG LLC
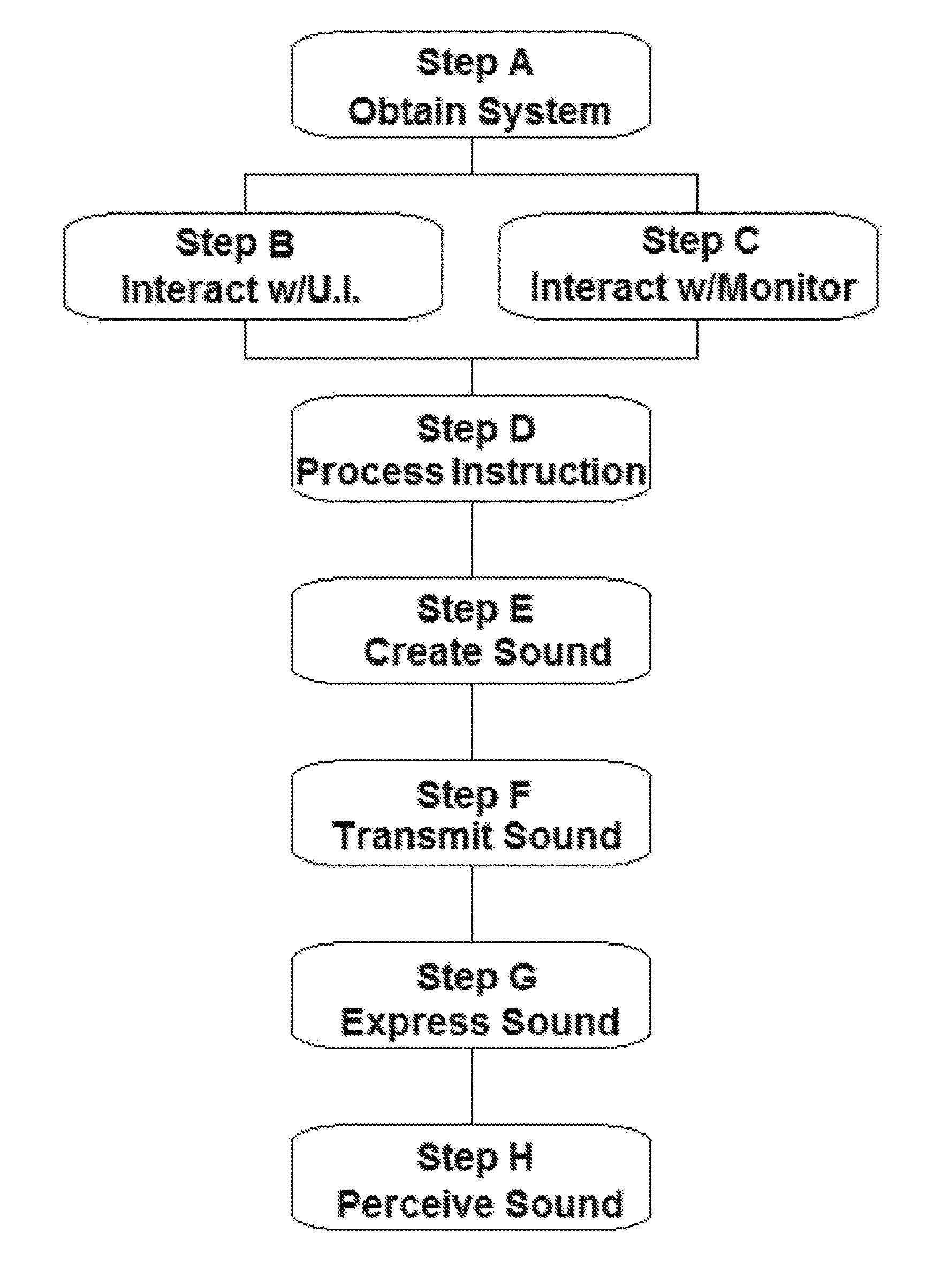
Method of music instruction
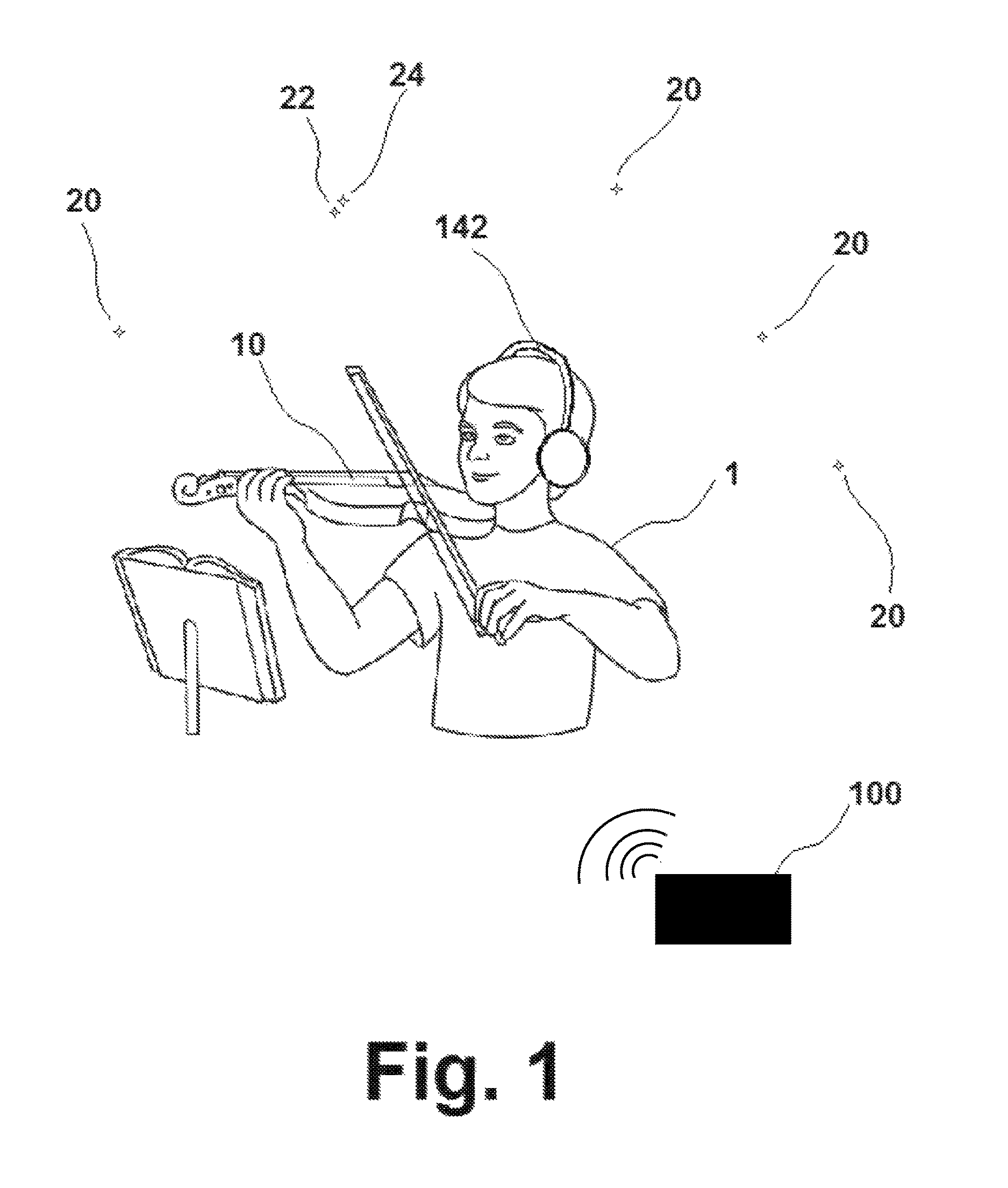
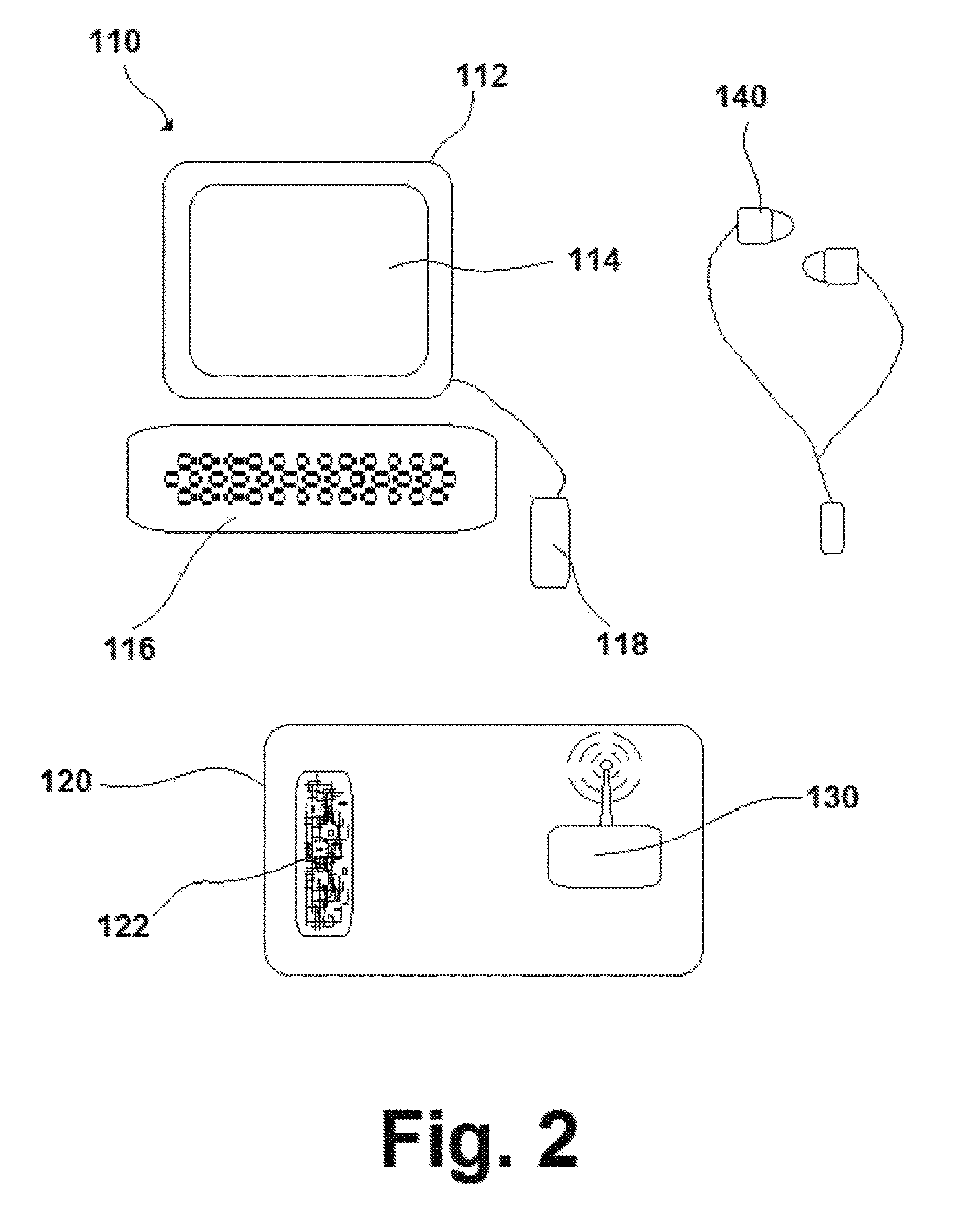
ActiveUS9551979B1Improve perceptionImprove cognitionElectrophonic musical instrumentsMetronomesThree-dimensional spaceComputer science
A method of music instruction utilizing a system capable of producing two or more sounds perceived by the user as originating in specific locations in three-dimensional space relative to the user, with the system comprising a user interface, a sound generator, a transmitter, and a monitoring device, and the method comprising the steps of obtaining the system; interacting with the user interface of the system to provide instruction to the sound generator; interacting with the monitoring device; generating the one or more sounds perceived to be emanating from some location in three-dimensional space based on the instruction provided; transmitting output signals to the monitoring device; and perceiving the sounds by means of the monitoring device, with the method being practiced at the same time that the user either plays or does not play a musical instrument.
Owner:DOWNEY PATRICK M
Multi-nutrient composition
ActiveUS20200230197A1Improve cognitionAdd additional massOrganic active ingredientsNervous disorderBlood cholesterolNutrition
A method of improving at least one of the following in an individual: lean mass, muscle strength, cognition, systemic inflammation levels, blood cholesterol levels, blood triglyceride levels and glucose tolerance is provided, comprising administering to the individual a multi-nutrient composition a protein, creatine, vitamin D, calcium and an n-3 fatty acid.
Owner:EXERKINE
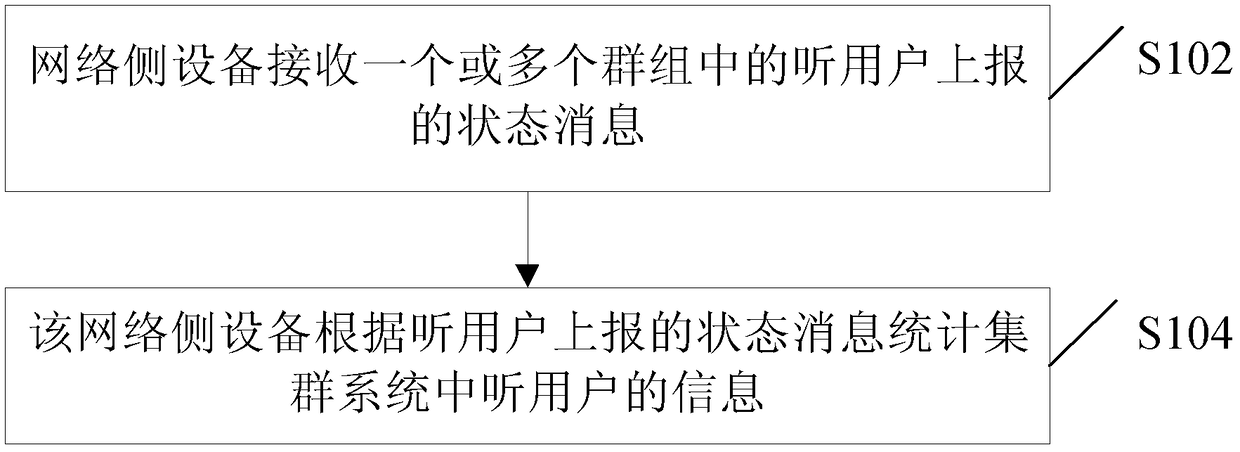
Statistical method, device, and system for listening to user information, and listening user terminal
ActiveCN103249008BImprove cognitionBroadcast service distributionRelevant informationComputer science
The invention discloses a statistical method, device and system for listener information and a listener terminal. The method comprises the following steps: status messages reported by listeners in one or more groups are received by a network side device; and listener information in a clustered system is gathered by the network side device according to the status messages reported by the listeners. According to the invention, the network side device receives the status messages reported by the listeners in one or more groups and gathers the listener information in the clustered system according to the status messages, so that the network side device in the clustered system can obtain the listener information of the groups the clustered system, the problem that relevant information of listeners in a cluttered system in the prior art cannot be obtained is solved, and the awareness of the clustered system on the listener information of the clustered system is improved.
Owner:SHANGHAI ZTE SOFTWARE CO LTD
Pre-natal beta-cryptoxanthin benefits children
PendingUS20220079888A1Increased cognition scoreGood effectHydrocarbon active ingredientsNervous disorderLycoperseneFine motor skill
Beta-cryptoxanthin administered to a pregnant woman has numerous benefits. It can decrease anxiety, and lessen the risk of her developing anxiety during her pregnancy. Further it can impart various benefits to her child: increased cognition, increased receptive language skills, fine motor skills, and gross motor skills. Also lycopene administered to the pregnant woman can increase expressive language in the child. Pre-natal supplements are also included in this invention.
Owner:AGENCY FOR SCI TECH & RES +1
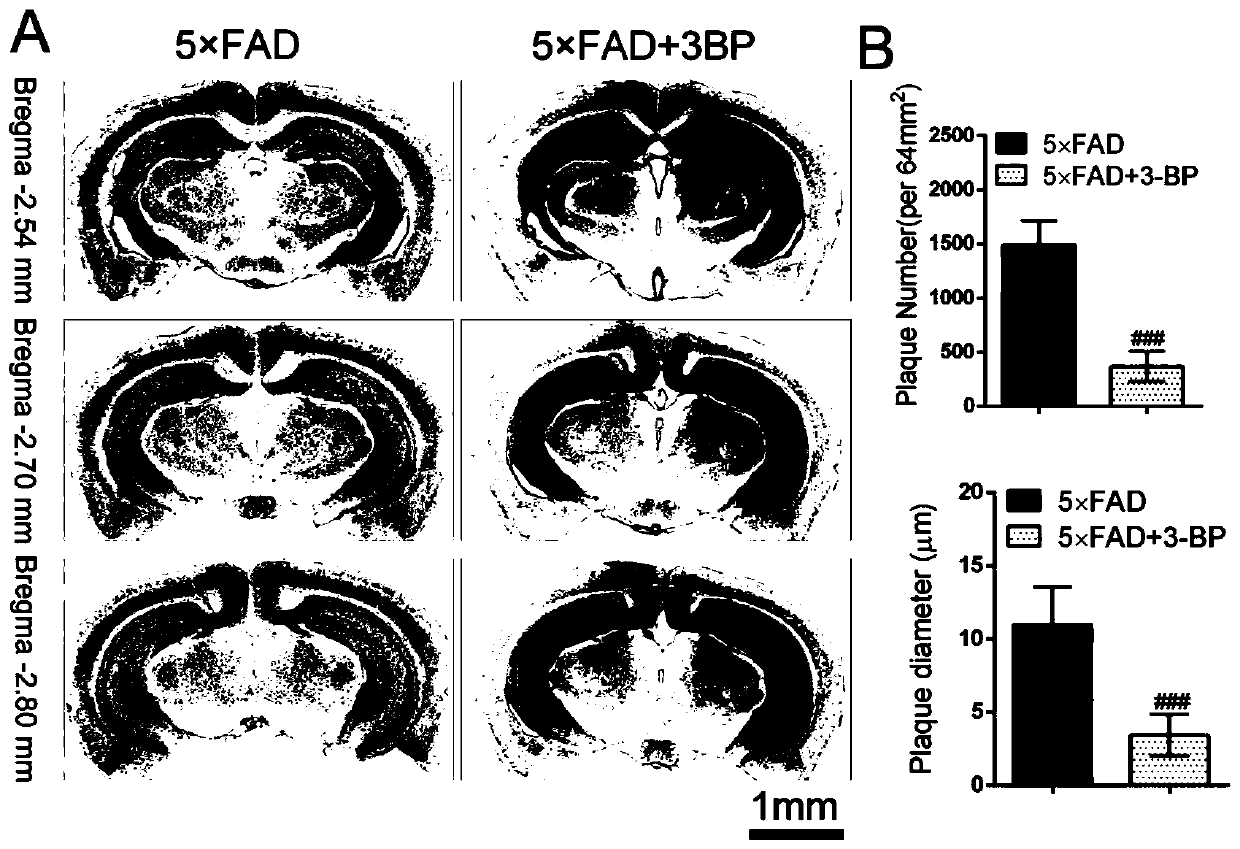
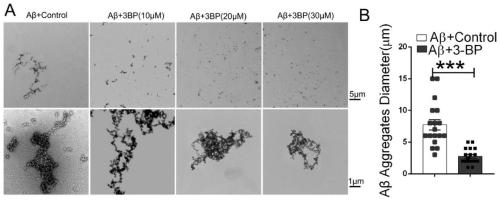
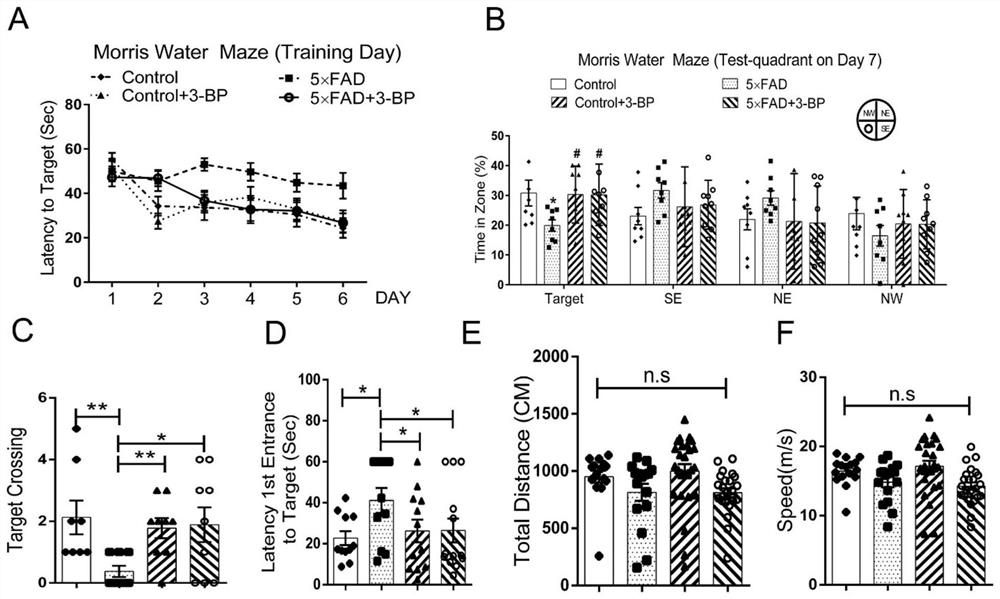
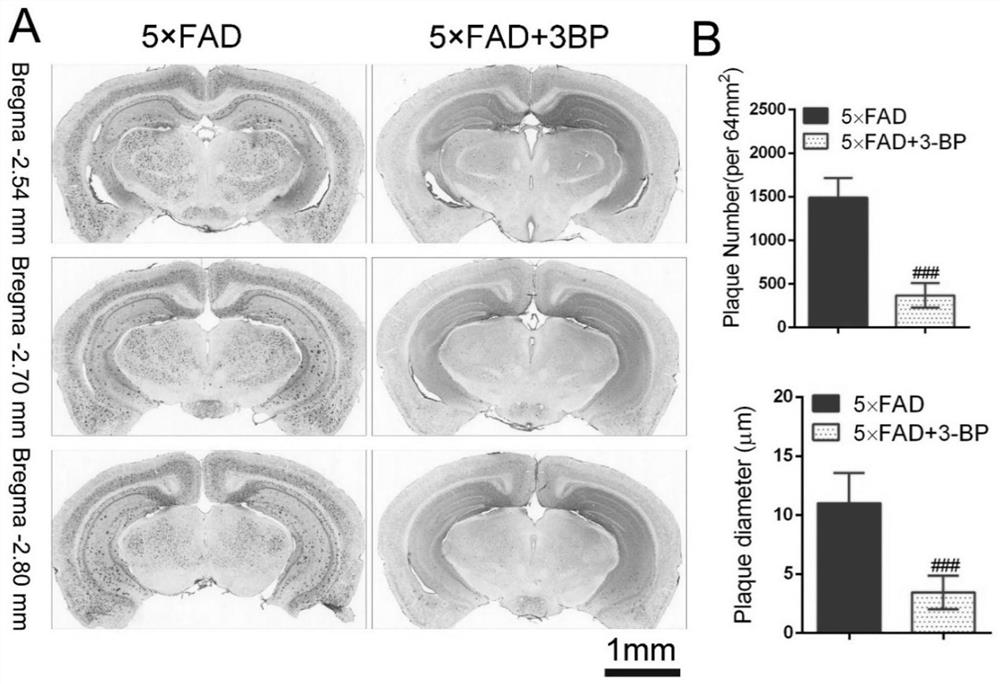
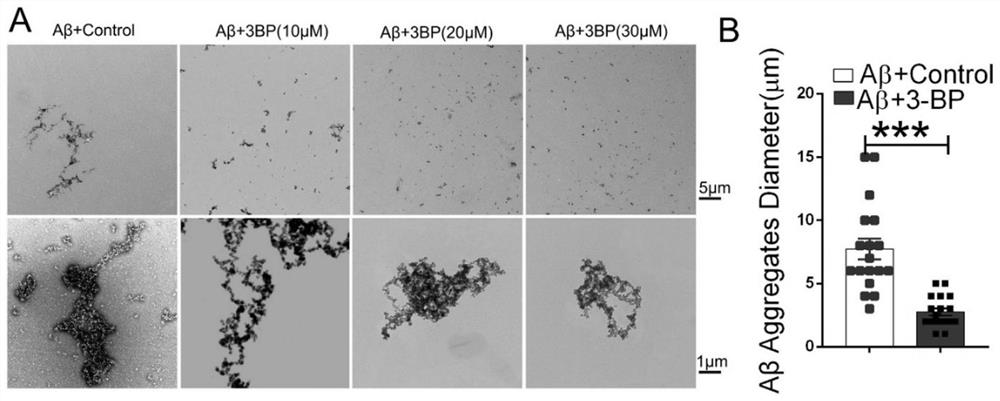
Application of hexokinase inhibitor to preparation of medicine for preventing and/or treating Alzheimer disease
ActiveCN111358784AImprove cognitionReduce aggregationNervous disorderAnhydride/acid/halide active ingredientsBromopyruvic acidPharmaceutical drug
The present invention discloses an application of a hexokinase inhibitor to preparation of a medicine for preventing and treating the Alzheimer disease. It is proposed that the hexokinase inhibitor, especially lonidamine and 3-bromopyruvic acid can improve the cognitive level of an Alzheimer disease model mouse, remove A beta and reduce A beta aggregation, the hexokinase inhibitor has the obviousanti-Alzheimer disease progression effect, and a basis is provided for the hexokinase inhibitor, especially the lonidamine and the 3-bromopyruvic acid to preparation of the medicine for preventing andor treating the Alzheimer disease.
Owner:XIAMEN UNIV
Maternal breast milk with natural bioactive nutraceutical extracts
PendingUS20220054577A1Improve efficacyEnhance intestinal functionMetabolism disorderUnknown materialsBiotechnologyEnzymatic hydrolysis
Disclosed are nutraceutical compositions and formulations, and methods of using bioactive nutraceutical compositions and formulations of enzymatically hydrolyzed stabilized rice bran extract for the improvement of maternal breast milk, and for the mitigation of chronic malnutrition in infants, wherein the infant is breast feeding from a lactating mother ingesting said nutraceutical compositions or formulations in accordance with a prescribed regimen.
Owner:INTERMARK PARTNERS STRATEGIC MANAGEMENT
Use of hexokinase inhibitors in the preparation of drugs for preventing and/or treating Alzheimer's disease
ActiveCN111358784BImprove cognitionReduce aggregationNervous disorderAnhydride/acid/halide active ingredientsBromopyruvic acidPharmaceutical drug
The present invention discloses an application of a hexokinase inhibitor to preparation of a medicine for preventing and treating the Alzheimer disease. It is proposed that the hexokinase inhibitor, especially lonidamine and 3-bromopyruvic acid can improve the cognitive level of an Alzheimer disease model mouse, remove A beta and reduce A beta aggregation, the hexokinase inhibitor has the obviousanti-Alzheimer disease progression effect, and a basis is provided for the hexokinase inhibitor, especially the lonidamine and the 3-bromopyruvic acid to preparation of the medicine for preventing andor treating the Alzheimer disease.
Owner:XIAMEN UNIV
Oxybutynin transdermal therapeutic system muscarinic agonist combination
ActiveUS20180050008A1Safety managementIncrease awarenessNervous disorderAerosol deliveryNervous systemHigh doses
Pharmaceutical compositions and combinations containing a muscarinic receptor antagonist, such as oxybutynin in a transdermal therapeutic system, and a muscarinic receptor agonist, optionally with an acetyl cholinesterase inhibitor, and methods of using the same for treatment of hypocholinergic disorders of the central nervous system such as Alzheimer type dementia. The respective pharmaceutical compositions and combinations of the present invention allow for safe administration of high doses of muscarinic receptor agonist, and improved efficacy of the muscarinic receptor agonist for treatment of hypocholinergic disorders of the central nervous system. The pharmaceutical compositions and combinations also allow for a maximum supply of acetylcholine to the central nervous system, when an acetyl cholinesterase inhibitor is used in combination with a muscarinic receptor antagonist and a muscarinic receptor agonist.
Owner:CHASE PHARMA CORP
Compositions and methods for treating neurodegenerative disorders with rifaximin
PendingUS20210186937A1Reduce ammonia levelsAltering gut floraNervous disorderSynthetic polymeric active ingredientsCytokineDegenerative disease
This invention relates generally to neurodegenerative diseases and conditions (e.g., Alzheimer's disease) characterized with higher than normal brain blood ammonia levels and / or higher than normal amounts of circulatory pro-inflammatory cytokines secreted by harmful gut bacteria. This invention further relates to methods and compositions for treating such neurodegenerative diseases and conditions with pharmaceutical compositions capable of reducing blood ammonia levels and / or reducing levels of circulatory pro-inflammatory cytokines secreted by harmful gut bacteria.
Owner:DUKE UNIV
Training equipment to improve the ability of cognition and memory and the muscle power of upper and lower limb and training method thereof
ActiveUS11452909B2Increase muscle strengthLow powerVideo gamesTeaching apparatusUpper limb muscleVirtual space
Owner:MAN&TEL
Treatment of cognitive disorders
InactiveUS20170119775A1Induce gastrointestinal toxicityConvenient treatmentNervous disorderEster active ingredientsCognitive diseasesCognition.knowledge
The technology provided herein relates to the novel use of compounds for improving cognition, concentration capacity, learning capacity and / or memory retentiveness, in particularly for the treatment and / or prophylaxis of cognitive, concentration capacity, learning capacity and / or memory retentiveness disorders.
Owner:ALGIAX PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com