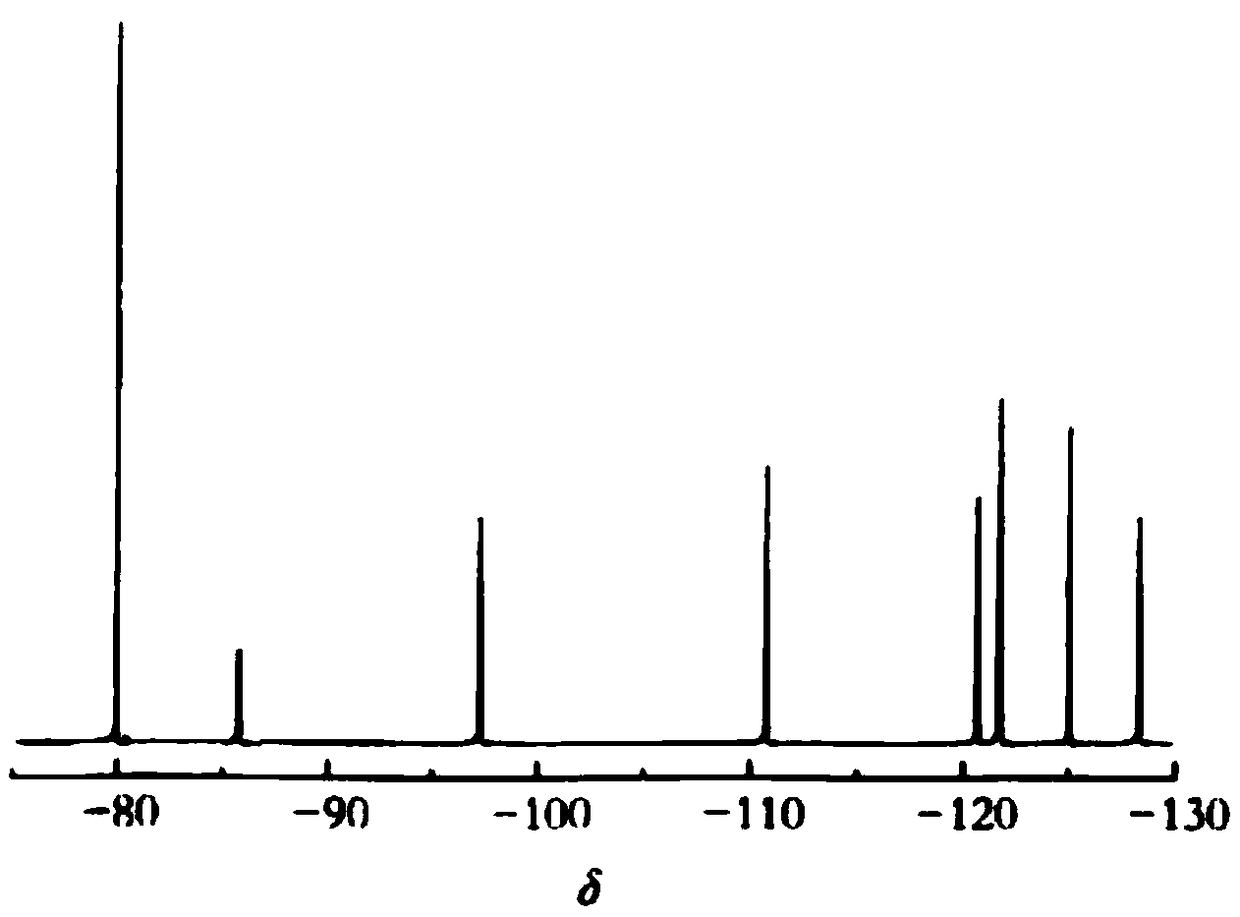
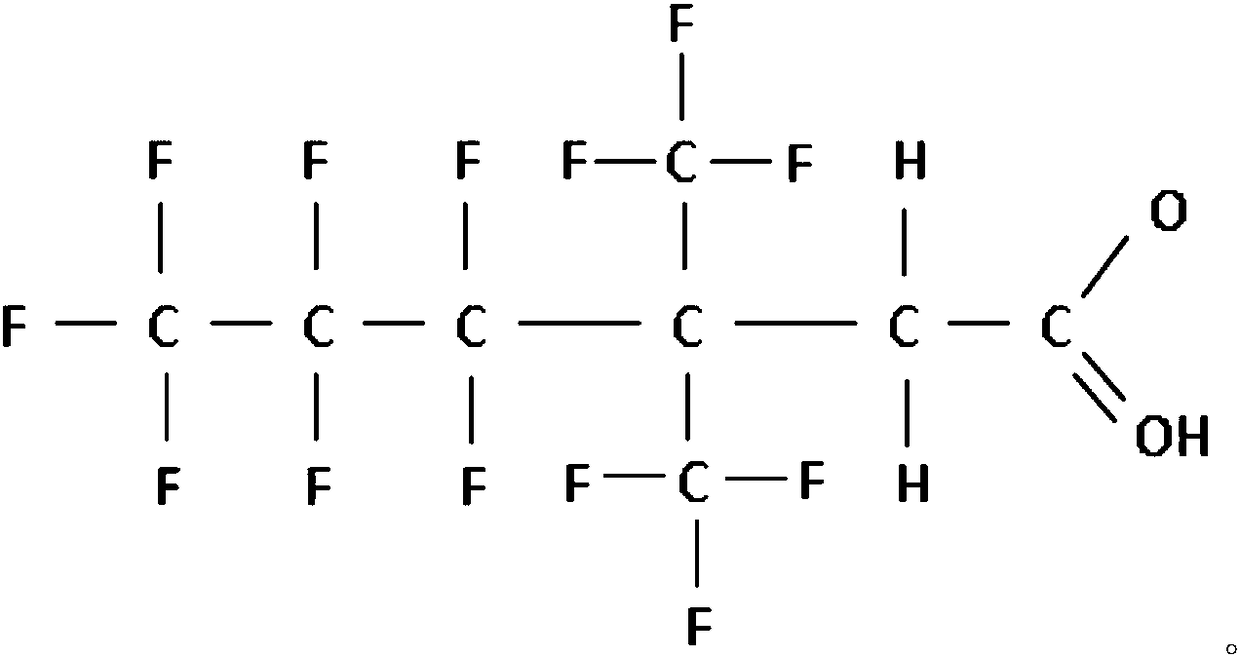
Ethylperfluorohexyl acetic acid and synthesizing method thereof
A synthetic method, perfluorohexyl technology, applied in chemical instruments and methods, carboxylate preparation, carboxylate preparation, etc., can solve the problems of high corrosion, highly toxic oxidant, high cost, etc., and achieve high heat resistance stability , high chemical stability, and easy industrial mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
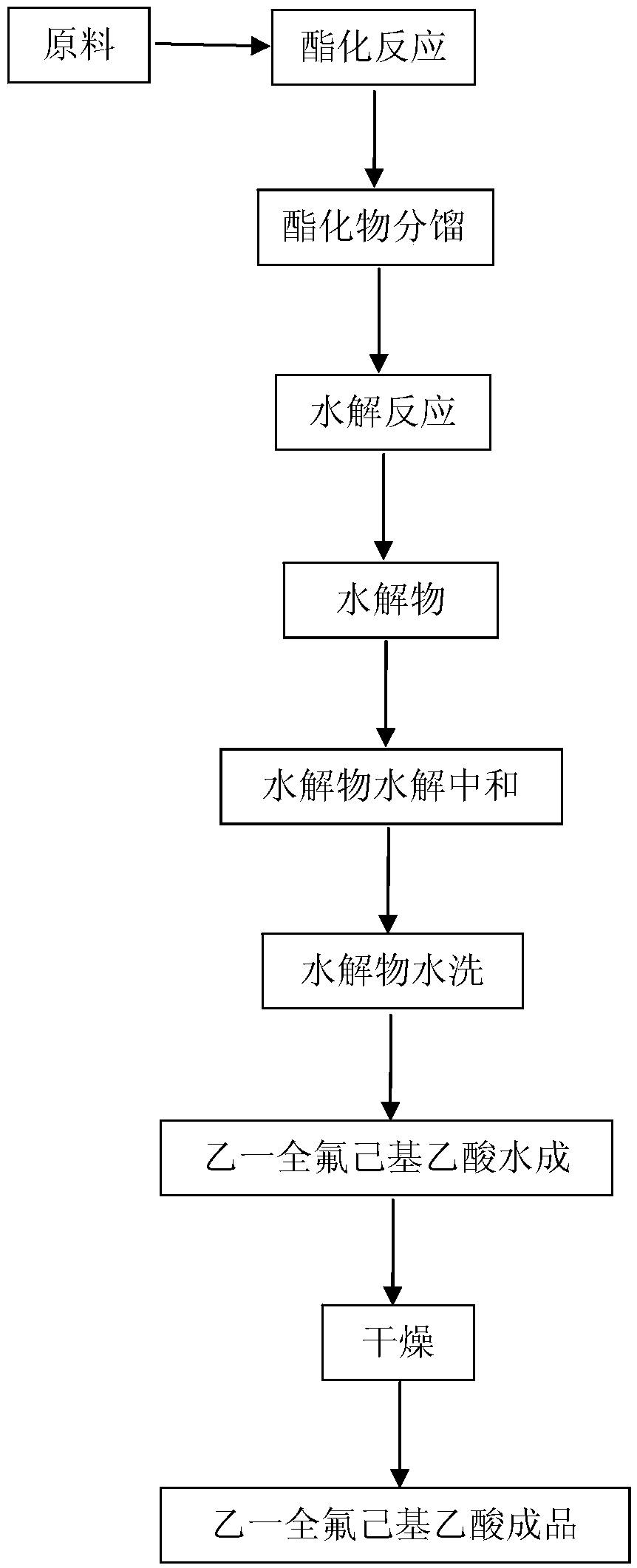
[0025] A kind of synthesis method of ethylene-perfluorohexylacetic acid provided by the embodiment of the present invention comprises the following steps:
[0026] (1) Mix hexafluoropropylene dimer D2, phenol, organic solvent and fluorine salt and heat to 50°C-70°C, then add BrCH in batches 2 CO 2 R, R includes -CH 3 or -CH 2 CH 3 , and then heating the formed mixed reaction system to 75° C. to 90° C. and keeping it warm for esterification reaction. After the reaction, the obtained reaction mixed system is lowered to room temperature;
[0027] (2) Fractional distillation is carried out to the reaction mixture system obtained in step (1), and the esterified product is separated therein;
[0028] (3) hydrolyzing the esterified product obtained in step (2) with a base to obtain a salt;
[0029] (4) neutralize the salt obtained in step (3) with an acid to obtain ethyl-perfluorohexylacetic acid.
[0030] In some embodiments, the step (1) specifically includes: mixing hexafluo...
Embodiment 1
[0051] Embodiment 1: the ethylene-perfluorohexylacetic acid synthetic method of the present embodiment comprises following specific steps:
[0052] (1) Esterification reaction
[0053] Raw materials include: C 6 f 12 (D2), methyl bromoacetate, DMF, potassium fluoride, phenol. The reaction formula is as follows:
[0054] C 6 f 12 +KF+BrCH 2 CO 2 R→C 6 f 13 CH 2 CO 2 R+KBr (R=CH 3 , CH 2 CH 3 )
[0055] The reaction temperature is 50-85°C. 40 parts by weight of methyl bromoacetate are pumped into the head tank through a diaphragm pump for subsequent esterification. 60 parts by weight of DMF, 6 parts by weight of phenol, and 50 parts by weight of polymer solution are pumped into the esterification kettle using a diaphragm pump. Put 30 parts by weight of potassium fluoride into the kettle and start stirring, turn on the electric heating jacket to start heating and set the temperature to 65°C. When the temperature of the material in the kettle rises to a certain te...
Embodiment 2
[0066] Embodiment 2: the synthetic method of the ethylene-perfluorohexylacetic acid of the present embodiment comprises the following concrete steps:
[0067] (1) Esterification reaction
[0068] Raw materials include: C 6 f 12 (D2), methyl bromoacetate, DMF, potassium fluoride, phenol. The reaction formula is as follows:
[0069] C 6 f 12 +KF+BrCH 2 CO 2 R→C 6 f 13 CH 2 CO 2 R+KBr (R=CH 3 , CH 2 CH 3 )
[0070] The reaction temperature is 50-85°C. 30 parts by weight of methyl bromoacetate are pumped into the head tank through a diaphragm pump for subsequent esterification, 40 parts by weight of DMF, 2 parts by weight of phenol, and 70 parts by weight of polymer solution are pumped into the esterification kettle using a diaphragm pump. Put 10 parts by weight of potassium fluoride into the kettle and start stirring, turn on the electric heating jacket to start heating and set the temperature to 70°C. When the temperature of the material in the kettle rises to a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com