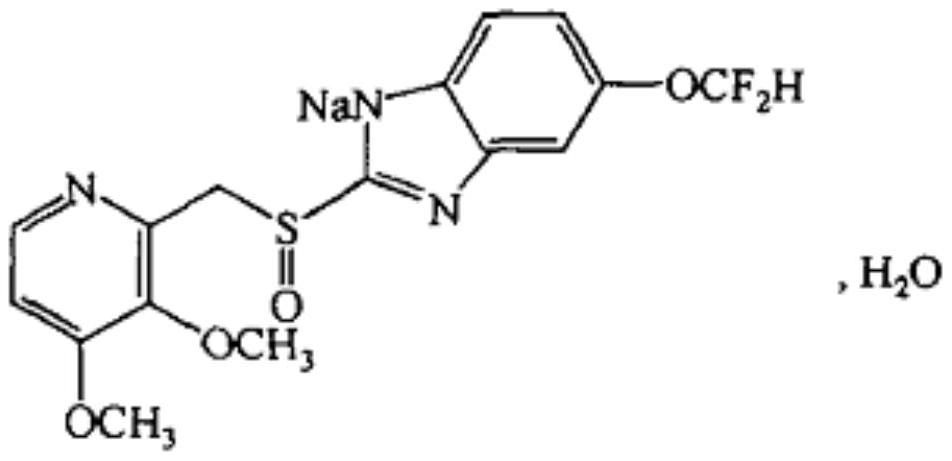
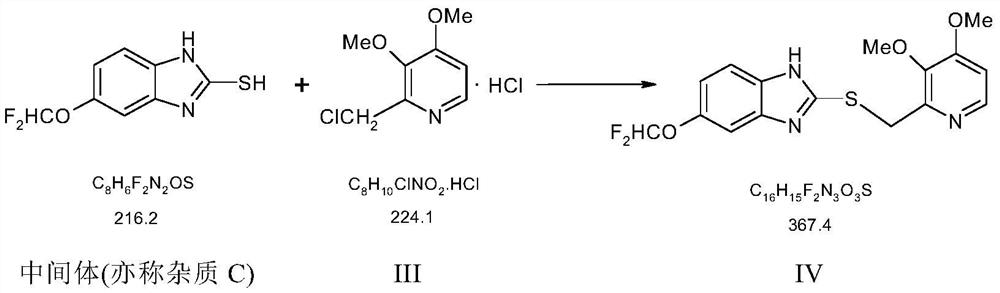
The preparation method of pantoprazole sodium and pantoprazole sodium
A technology of pantoprazole sodium and benzimidazole, applied in the field of medicine, can solve the problems of complicated steps, many side reactions, excessive C content of impurities and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Embodiment 1: Preparation of 2-chloromethyl-3,4-dimethoxypyridine hydrochloride (III)
[0169]
[0170] Add 9.22kg of toluene, add 1.5kg of 2-hydroxymethyl-3,4-dimethoxypyridine at room temperature, cool down to 3°C-10°C, add 1.63kg of chloride within 1-2 hours at 3-10°C After the addition of sulfoxide, keep stirring at 5-10°C for 1 hour, heat up to 35-40°C within 1 hour, and keep warm at 35-40°C for 4 hours. TLC detection After the reaction is completed, the temperature is controlled at 35-40°C, vacuum distilled thionyl chloride for 3-4 hours, and 9kg of toluene is added, the temperature is lowered to 20-25°C, centrifugal suction filtration, 0.64L toluene washes the filter cake, drains, 50 Blast drying at -55°C for 6-8 hours to obtain 2-chloromethyl-3,4-dimethoxypyridine hydrochloride (III).
Embodiment 2
[0171] Example 2: Preparation of 5-difluoromethoxy-2-[(3,4-dimethoxy-2-pyridyl)methyl]thio-1H-benzo Imidazole (IV)
[0172]
[0173] Add 1.0kg of 2-chloromethyl-3,4-dimethoxypyridine hydrochloride (III) to 6.0kg of purified water at 25°C, stir at 25°C to 30°C for 30 minutes, and filter with suction, 2.0kg Wash the filter paper with purified water to obtain filtrate A (stand-by);
[0174] Dissolve 0.7857kg of sodium hydroxide in 1.4293kg of purified water at room temperature, raise the temperature to 40°C, and add 1.0715kg of 5-difluoromethoxy-2-mercapto-1H-benzimidazole in batches within 30 minutes. Add the filtrate A dropwise at 40-45°C within 2-2.5 hours, keep the reaction at 48-52°C for 6 hours, TLC detects that it is below the limit; slowly cool down to 15°C, keep warm and crystallize at 13-17°C for 3-6 hours, Suction filtration, the solid was rinsed with 1.46kg of purified water, drained, and air-dried at 40-45°C for 10 hours to obtain crude product IV;
[0175] ...
Embodiment 21
[0176] Example 21: Preparation of 5-difluoromethoxy-2-[(3,4-dimethoxy-2-pyridyl)methyl]thio-1H-benzo Imidazole (IV)
[0177] This embodiment provides another method for preparing 5-difluoromethoxy-2-[(3,4-dimethoxy-2-pyridyl)methyl]thio-1H-benzimidazole (IV) method.
[0178] Add 2500ml of water and 204g of NaOH into the glass reactor and stir to dissolve, then add a mixture of 4000g of methanol and mercapto (5-difluoromethoxy-2mercapto-1H-benzimidazole, 500g). After heating to 40°C, add pyridine hydrochloride (2-chloromethyl-3,4-dimethoxypyridine hydrochloride, 500g), heat up to 50-55°C and react for 3.5 hours. After the reaction, release the reaction Liquid, use a rotary evaporator to steam methanol under reduced pressure, control the internal temperature to not exceed 50°C, evaporate as much as possible, then transfer the material to a stirred reaction tank, add 3000ml of dichloromethane, fully stir and dissolve, let stand to separate layers, and separate The aqueous l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com