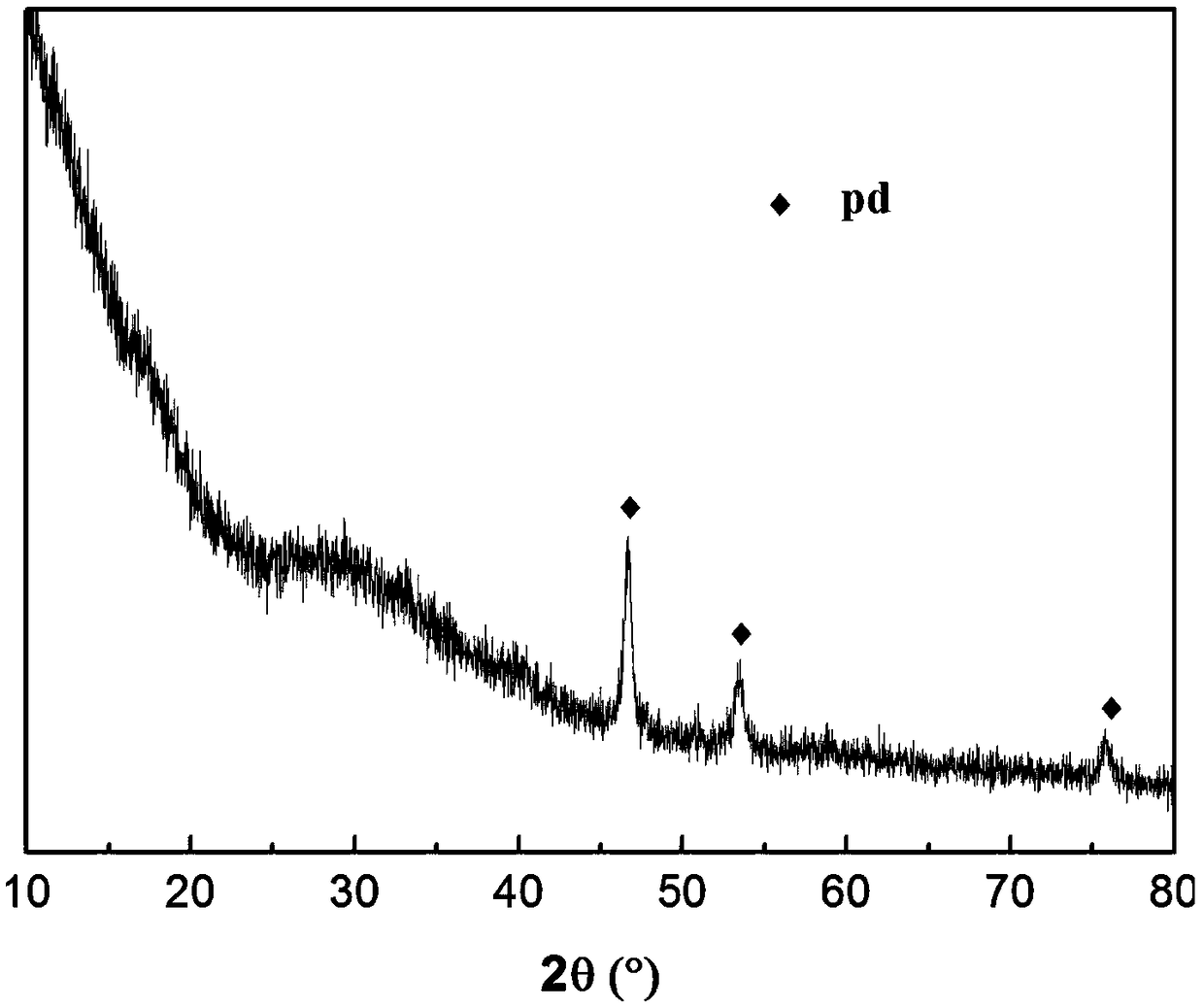
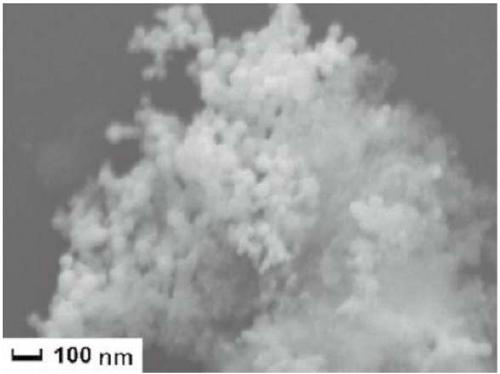
MCM-41/Sn-Pd catalyst as well as preparation method and application thereof
A technology of MCM-41 and catalyst, applied in the field of material preparation, can solve the problems of unfriendly environment and high cost, and achieve the effect of high catalyst activity and high dosage activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Preparation of template agent: Weigh 2.77g cetyltrimethylammonium bromide (CTAB) as template agent and surfactant at room temperature, dissolve 120g of deionized water and add 10.5ml of ammonia water dropwise during vigorous stirring to prepare Get a template.
[0024] (2) Preparation of MCM-41 / Sn: Weigh 1.17g of crystalline tin chloride (SnCl 4 ·5H 2 O) Dissolve in 11.2ml of tetraethylorthosilicate (TEOS), so that the molar ratio of silicon element to tin element in the mixed solution is 15. Stir the template agent vigorously for 10 minutes, then slowly drop the mixed solution into the template for 15 minutes. The white colloid was continuously stirred vigorously for 1 hour, transferred to a stainless steel autoclave with a polytetrafluoroethylene liner, and crystallized in an oven at 110°C for 48 hours. Then, it was repeatedly washed with deionized water and absolute ethanol, and dried at 60° C. for 16 hours to obtain a white molecular sieve powder.
[0025] (...
Embodiment 2
[0032] Same as Example 1, only change the 1,2-propanediol solution in step (5) of Example 1 to 0.02Mmol / L and 0.08Mmol / L respectively. The results obtained are shown in Table 1. The results show that with the increase of the concentration of 1,2-propanediol, the conversion rate of 1,2-propanediol increases, and the selectivity of pyruvic acid also increases slightly, indicating that the catalyst has a higher activity at a certain substrate concentration under experimental conditions. Both 1,2-propanediol and pyruvic acid maintain high conversion and selectivity within the range.
[0033] The impact of different 1,2-propanediol concentrations on the conversion rate of the final raw material and the selectivity of the reaction product in table 1
[0034] 1,2-propanediol concentration (Mmol / L)
Embodiment 3
[0036] Same as in Example 1, except that the reaction temperatures in step (5) of Example 1 were changed to 100°C and 120°C respectively, and then the catalyzed 1,2-propanediol reaction was carried out. The results obtained are shown in Table 2. The results show that with the increase of reaction temperature, the conversion rate of 1,2-propanediol and the selectivity of pyruvic acid increase, indicating that the catalyst has higher activity for 1,2-propanediol and pyruvic acid in a certain reaction temperature range under experimental conditions. Pyruvate maintained high conversion and selectivity.
[0037] The influence of table 2 different reaction temperatures on the conversion rate of final raw material and the selectivity of reaction product
[0038] Reaction temperature (°C)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com