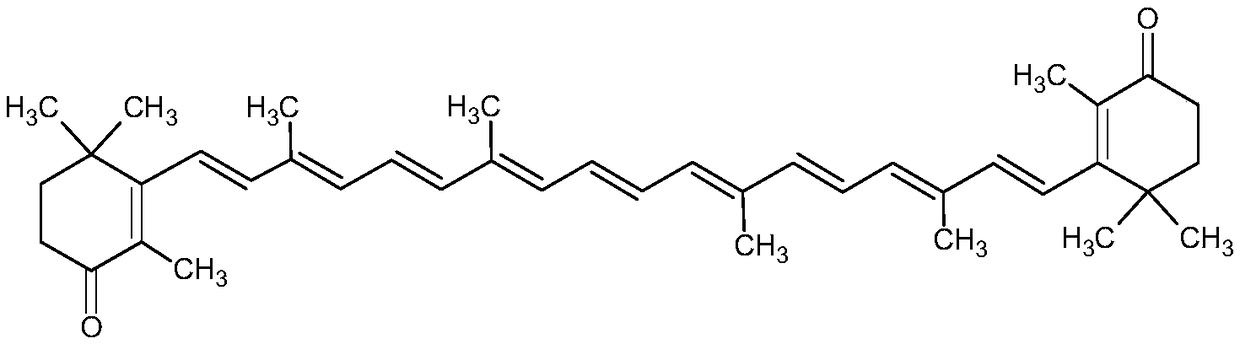
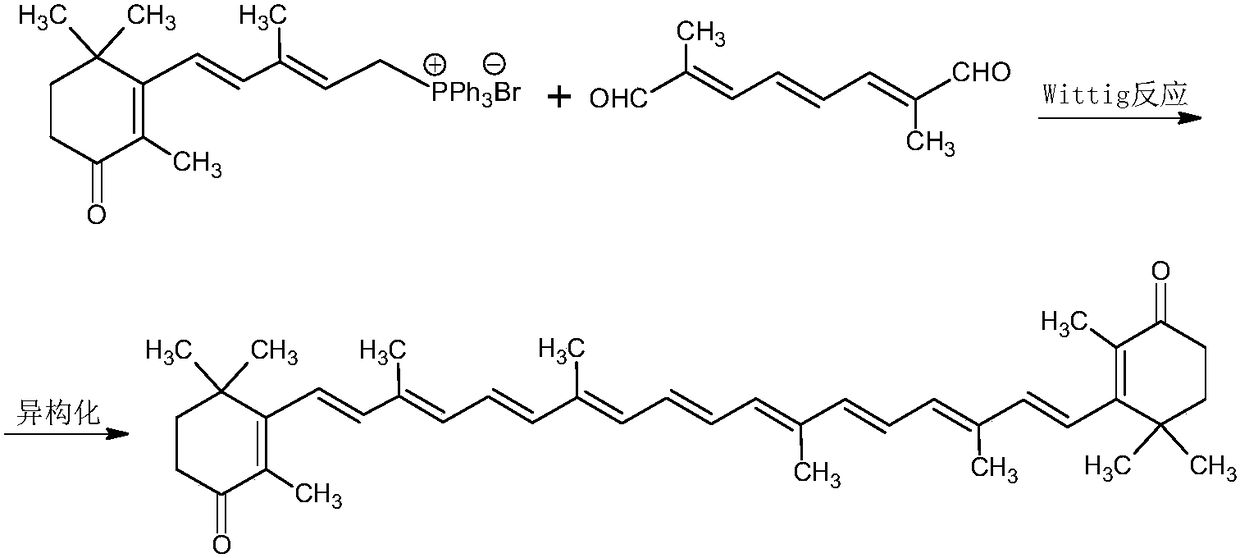
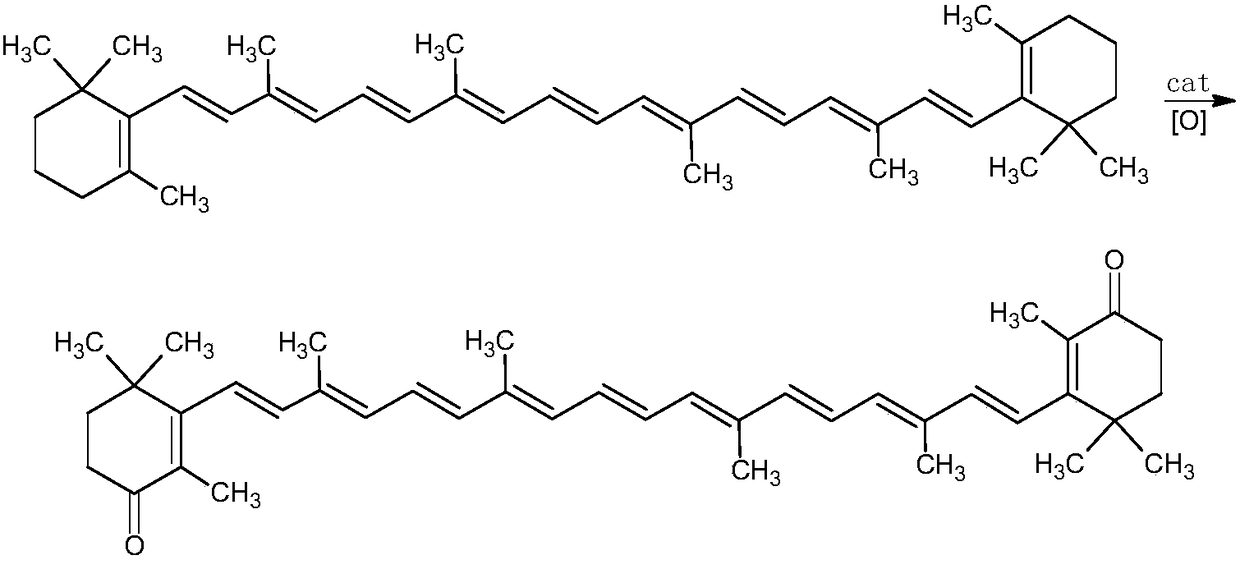
Method for preparing canthaxanthin
A technology of cantharidin and oxidant, applied in the directions of organic chemistry, organic chemistry, etc., can solve the problems of large amount of oxidant and low yield of cantharidin, and achieve the effects of shortening reaction time, reducing dosage and improving yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0033] The method of dissolving the β-carotene, the oxidizing agent I and the phase transfer catalyst in the organic solvent is not particularly limited in the present invention, and the above three substances can be added into the organic solvent in any order for dissolution. According to a specific embodiment of the present invention, the method of dissolving the β-carotene, the oxidizing agent I and the phase transfer catalyst in the organic solvent is to dissolve the β-carotene in the organic solvent first, and then add the oxidizing agent I and the phase transfer catalyst. The phase transfer catalyst is dissolved.
[0034] In the present invention, the temperature of the system needs to be controlled at 0-15° C. when adding the oxidizing agent I, that is, after the β-carotene, the oxidizing agent I and the phase transfer catalyst are dissolved in the organic solvent, the temperature of the system is higher than 15° C. °C or lower than 0 °C, the temperature of the system n...
Embodiment 1
[0046] Add 6g beta-carotene (99%, 0.01106mol) and 120ml methylene dichloride in 500ml four-necked flask, add 8g hydrogen peroxide aqueous solution (10%w / w, 0.02352mol) and 0.03g tetrabutylammonium bromide, Vacuumize and replenish nitrogen three times, cool down to 0-5°C, add 27.6g of sodium hypochlorite aqueous solution (available chlorine content is 1.5wt%, 0.01166mol, pH value is adjusted to 7) which is pre-cooled to 5°C very slowly, and the temperature of the dropping process is Control at 0-5°C, keep warm at this temperature for 6 hours after the dropwise addition, stand and separate to obtain an oil layer and a water layer, extract the water layer once with 30ml dichloromethane, combine the extracted dichloromethane layer and the oil layer with 100ml Wash once with 2% w / v sodium sulfite aqueous solution, once with 100ml of water, concentrate the oil layer to dryness, add 50ml of ethanol, perform isomerization reaction at 70°C for 8 hours, lower to normal temperature, filte...
Embodiment 2
[0048] The 8g hydrogen peroxide aqueous solution (10%w / w, 0.02352mol) in embodiment 1 is replaced by 6.41g tert-butyl alcohol peroxide aqueous solution (70%w / w, 0.04979mol), dropwise temperature and follow-up insulation temperature All are replaced with 5~10 ℃, all the other are with embodiment 1, obtain 5.70g cantharidin, content is 95.23%, and molar yield is 87.02%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com