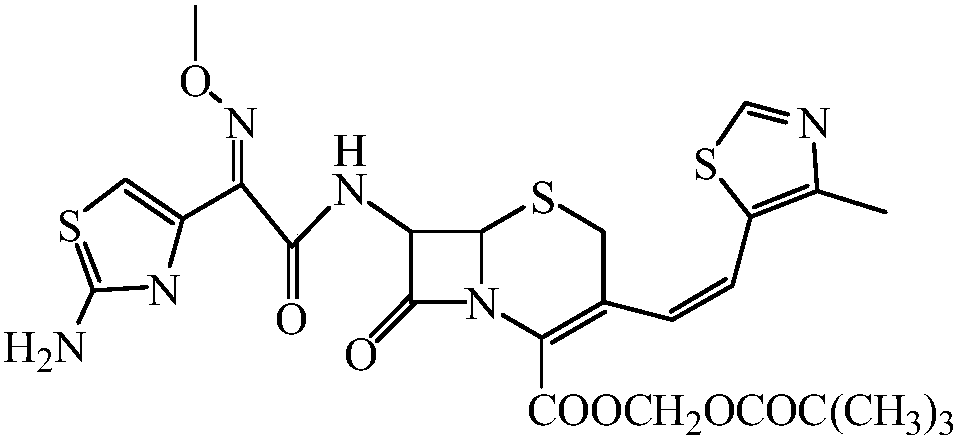
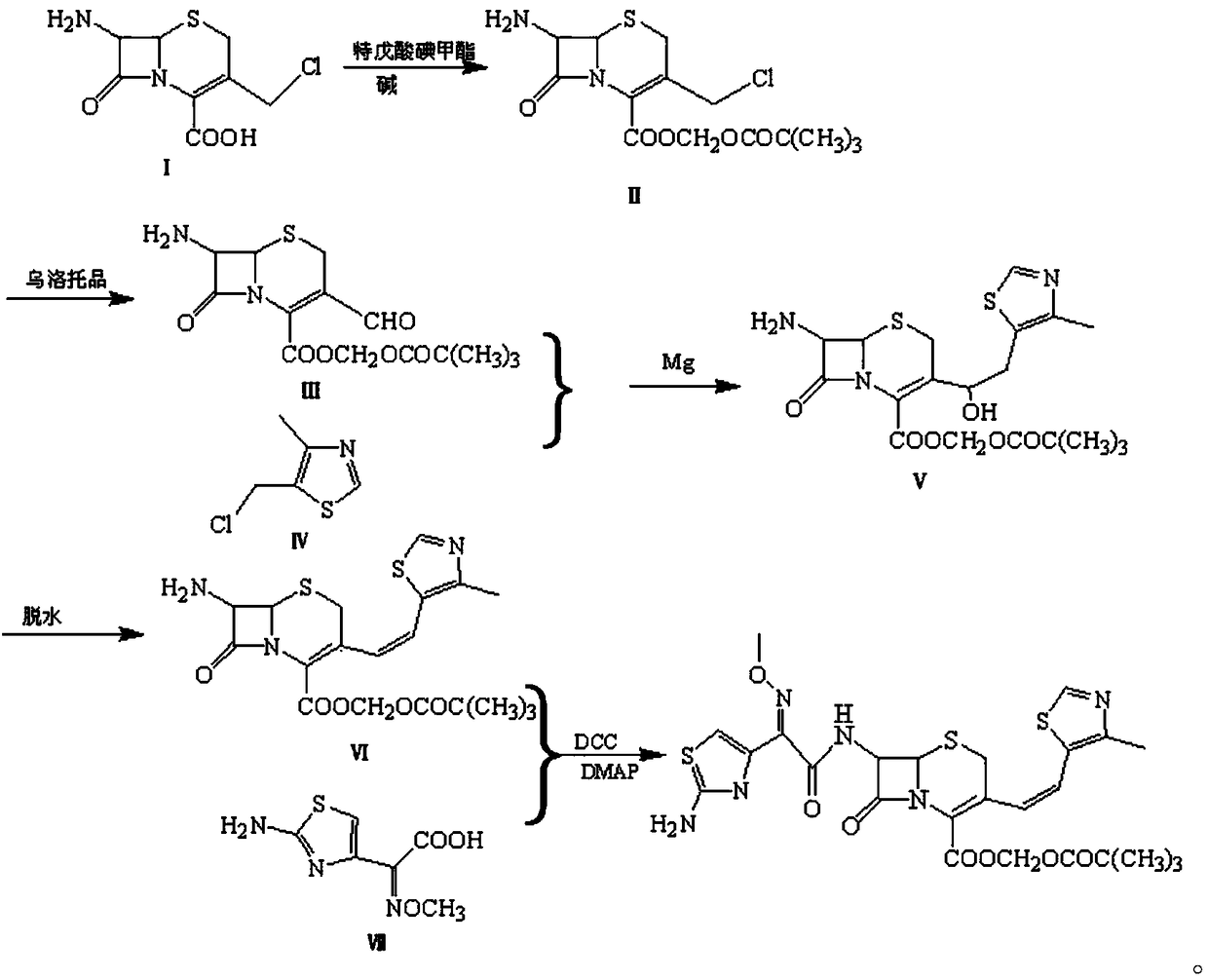
Preparation method of cefditoren pivoxil
A technology of cefditoren pivoxil and cephem, which is applied in the field of antibiotic preparation, can solve the problems of poor configuration selectivity, long time consumption, and poor utilization of solid waste triphenylphosphine, and achieve low cost and high selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A kind of preparation method of cefditoren pivoxil, concrete steps are as follows:
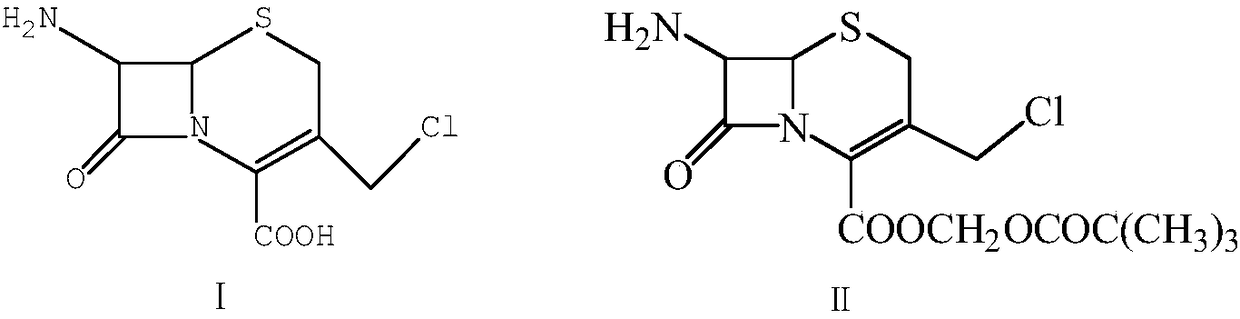
[0024] Dissolve 24.9g of 7-amino-3-chloromethyl-3-cephem-4-carboxylic acid (I) in toluene, add 4g of NaOH, then add 29g of iodomethyl pivalate, reflux for 1.5h, and react After the end, the reaction solution was washed with water, and the solvent was evaporated to obtain 34.8 g of 7-amino-3-chloromethyl-3-cephem-4-carboxylic acid trimethylacetoxymethyl ester (II); Add acetic anhydride to dissolve, add 16.8g urotropine, react at 30°C for 2h, after the reaction, pour the reaction solution into 10% aqueous sodium hydroxide solution, stir for 5min, extract with ethyl acetate, evaporate the organic phase Solvent to obtain 31.7g of compound (Ⅲ); soak 2.7g of magnesium in tetrahydrofuran, then dissolve 14.8g of 4-methyl-5-chloromethylthiazole (Ⅳ) in tetrahydrofuran, drop into the tetrahydrofuran system soaked in magnesium , heating to reflux to initiate the reaction, and reflux for 1 hour aft...
Embodiment 2
[0026] A kind of preparation method of cefditoren pivoxil, concrete steps are as follows:
[0027] Dissolve 24.9g of 7-amino-3-chloromethyl-3-cephem-4-carboxylic acid (I) in toluene, add 6g of NaOH, then add 36g of iodomethyl pivalate, reflux for 1h, and the reaction ends After the reaction liquid was washed with water, the solvent was evaporated to obtain 35.5 g of 7-amino-3-chloromethyl-3-cephem-4-carboxylic acid trimethylacetoxymethyl ester (Ⅱ); Dissolve in acetic anhydride, add 20g urotropine, react at 20°C for 3h, after the reaction, pour the reaction solution into 10% aqueous sodium hydroxide solution, stir for 5min, extract with ethyl acetate, evaporate the organic phase to remove the solvent to obtain Compound (Ⅲ) 32.4g; Soak 2.8g magnesium in tetrahydrofuran, then dissolve 22g 4-methyl-5-chloromethylthiazole (Ⅳ) in tetrahydrofuran, drop into the tetrahydrofuran system soaked in magnesium, heat to reflux Initiate the reaction, reflux the reaction for 1 hour after drop...
Embodiment 3
[0029] A kind of preparation method of cefditoren pivoxil, concrete steps are as follows:
[0030] Dissolve 24.9g of 7-amino-3-chloromethyl-3-cephem-4-carboxylic acid (I) in toluene, add 6g of NaOH, then add 31g of iodomethyl pivalate, reflux for 1h, and the reaction ends After the reaction liquid was washed with water, and the solvent was distilled off, 35.0 g of 7-amino-3-chloromethyl-3-cephem-4-carboxylic acid trimethylacetoxymethyl ester (II) was obtained; (II) was added Dissolve in acetic anhydride, add 18g urotropine, react at 20°C for 3h, after the reaction, pour the reaction solution into 10% aqueous sodium hydroxide solution, stir for 5min, extract with ethyl acetate, evaporate the organic phase to remove the solvent to obtain Compound (Ⅲ) 32.1g; soak 2.8g of magnesium in tetrahydrofuran, dissolve 19g of 4-methyl-5 chloro-methylthiazole (Ⅳ) in tetrahydrofuran, drop into the tetrahydrofuran system soaked in magnesium, and heat to reflux to initiate the reaction. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com