A kind of coupling synthesis method of 4,4,4-trifluorobutanol
A technology of trifluorobutanol and cross-coupling reaction, applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxy compounds, etc., can solve the problems of low yield of synthetic routes, unfavorable industrialization and application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a kind of preparation method of 4,4,4-trifluorobutanol, comprising the following steps:
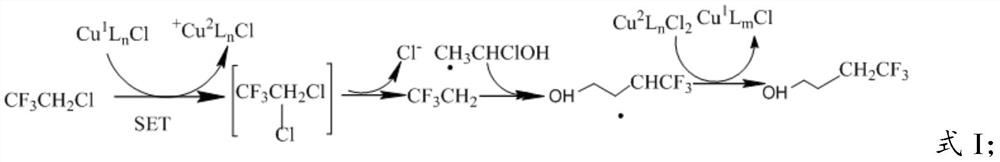
[0027] In the presence of a catalyst, mixing 2,2,2-trifluoro-1-haloethane and 1-chloroethanol in a solvent to perform a cross-coupling reaction to obtain 4,4,4-trifluorobutanol;
[0028] The catalyst is a copper salt and an organic ligand;
[0029] The copper salt is cuprous halide and / or cupric chloride; the organic ligand is 2,2'-bipyridine, tetramethylethylenediamine and 4,4-di-tert-butyl-2,2,- One or more of bipyridines.
[0030] In the present invention, the catalyst is preferably added to the reaction device now, and nitrogen gas is introduced, and then 2,2,2-trifluoro-1-haloethane, 1-chloroethanol and solvent are added to react to obtain 4,4,4 - Trifluorobutanol.
[0031] In the present invention, the catalyst is a copper salt and an organic ligand, and the organic ligand can complex the copper salt in the catalyst to promote the catalytic reaction....
Embodiment 1
[0045]Add CuI (0.5mmol) and 2,2'-bipyridine (1.0mmol) into a 5mL Schlenk tube, pump nitrogen three times, and add 2mL THF, 2,2,2-trifluoro-1- Chloroethane (10mmol) and 1-chloroethanol (5mmol), after adding the materials, stopper the plug, and heat in an oil bath at 50°C for 8h. After the reaction, the solid was removed by filtration, and then the filtrate was washed with saturated brine, and the organic phase and the water phase were separated with a separatory funnel. The organic phase was extracted with dichloromethane, dried over anhydrous sodium sulfate, and the combined filtrate was vacuum rotary evaporated. The product was separated by column chromatography with a yield of 80%.
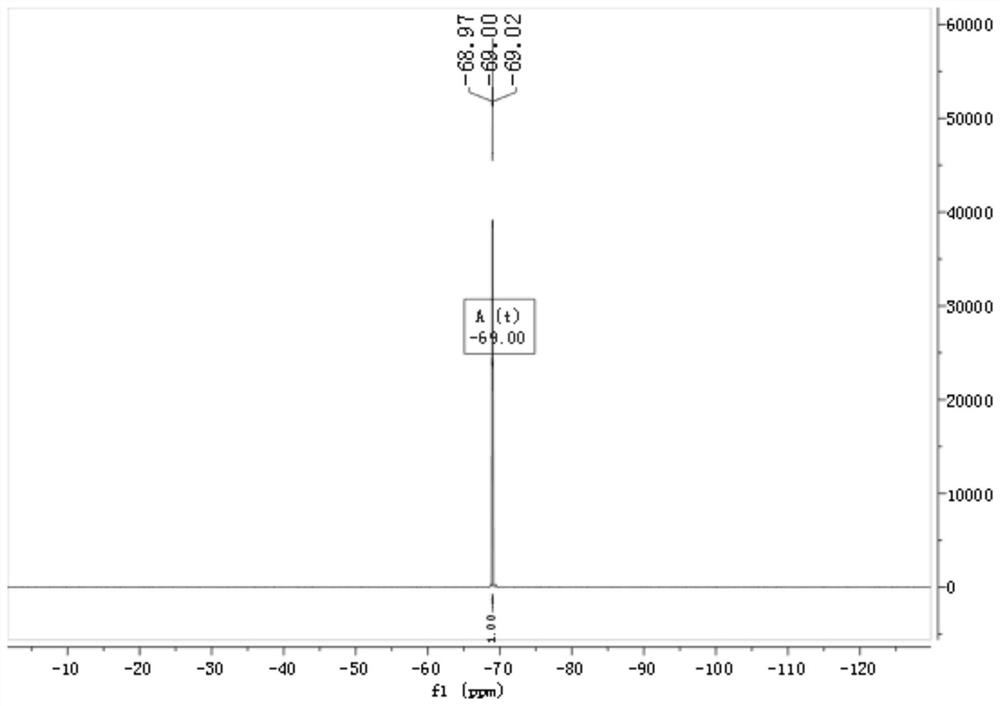
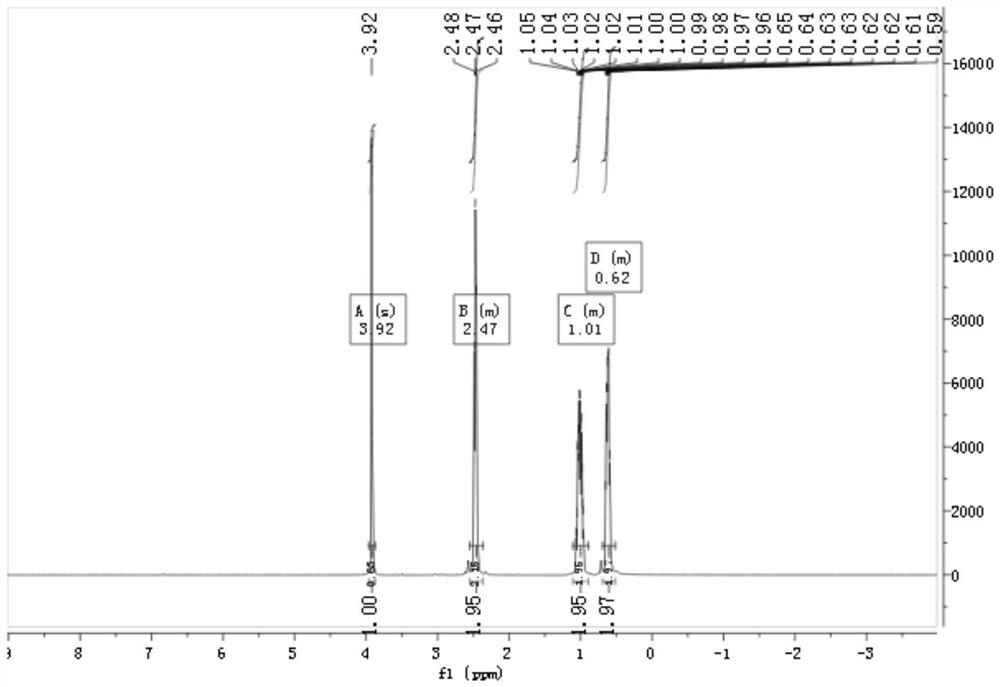
[0046] The proton and fluorine spectra of the product in this example are as follows Figure 1~2 shown by figure 1 with figure 2 It can be seen that the product prepared in this example is 4,4,4-trifluorobutanol.
Embodiment 2
[0048] Use CuCl instead of CuI to conduct experiments, add CuCl (0.5mmol) and 2,2'-bipyridine (1.0mmol) to a 5mL Schlenk tube, pump nitrogen three times, and add 2mL THF, 2,2, 2-Trifluoro-1-chloroethane (10mmol) and 1-chloroethanol (5mmol) were added, plugged with a stopper, and heated in an oil bath at 50°C for 8h. After the reaction, the solid was removed by filtration, and then the filtrate was washed with saturated brine, and the organic phase and the aqueous phase were separated with a separatory funnel. The organic phase was extracted with dichloromethane, dried over anhydrous sodium sulfate, and the combined filtrate was vacuum rotary evaporated, and the column The product was isolated by chromatography in a yield of 61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com